5- Alcohols
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
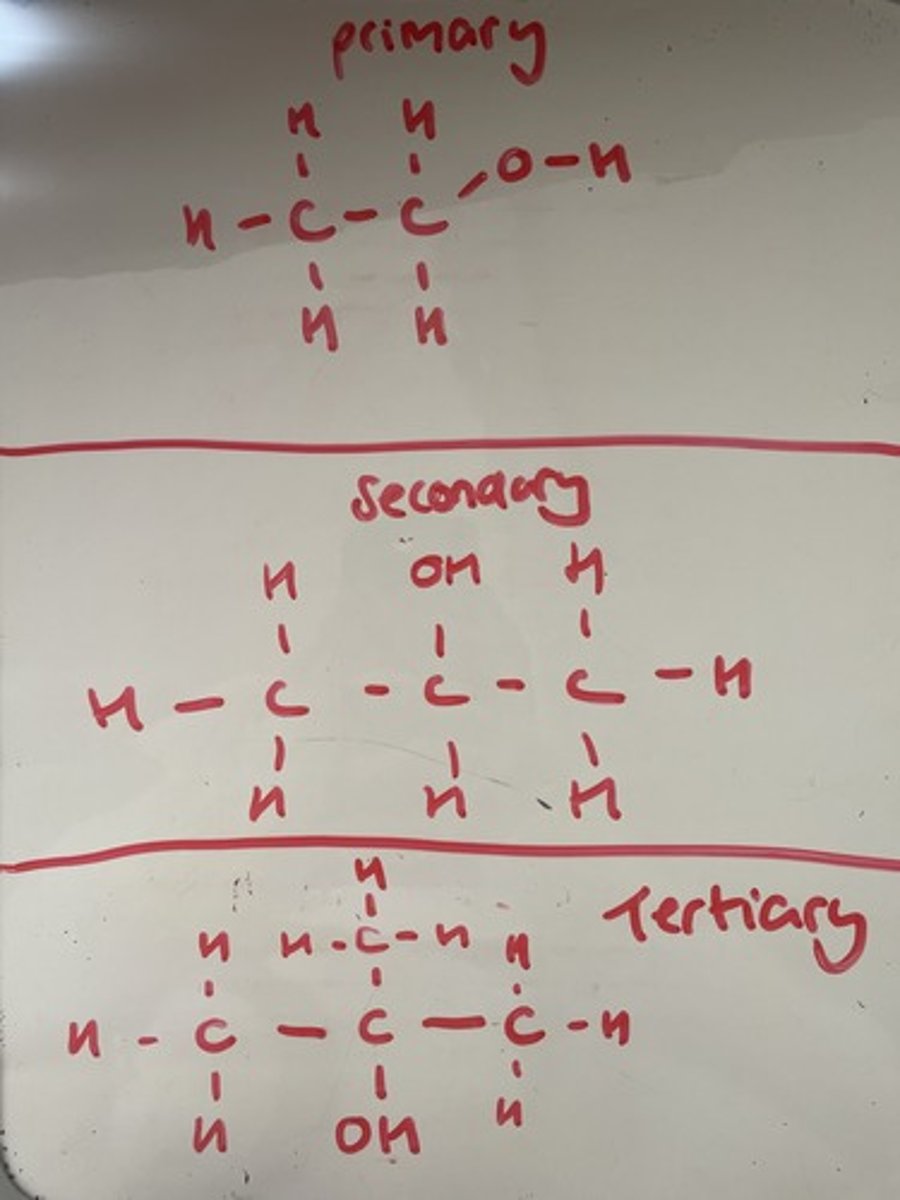
What is a primary, secondary and tertiary alcohol?
Primary - one carbon attached
Secondary - two carbons attached
Tertiary - three carbons attached
Solubility of alcohols
Generally soluble, the larger the hydrocarbon chain attached to the OH group, the less soluble in water.
The hydrogen on the hydroxyl group bonds with the lone pair on the oxygen of water this is why its soluble
Why do alcohols have low volatility? (doesn't evaporate readily)
Strong hydrogen bonding within the alcohol, less likely to break and hence evaporate
Boiling points of alcohols
The larger the Mr (hydrocarbon chain) the higher the boiling point as there are larger van De Waals forces
Uses of alcohols
-Chemical feedstocks
-Disinfectants
-Drinks
-Antifreeze
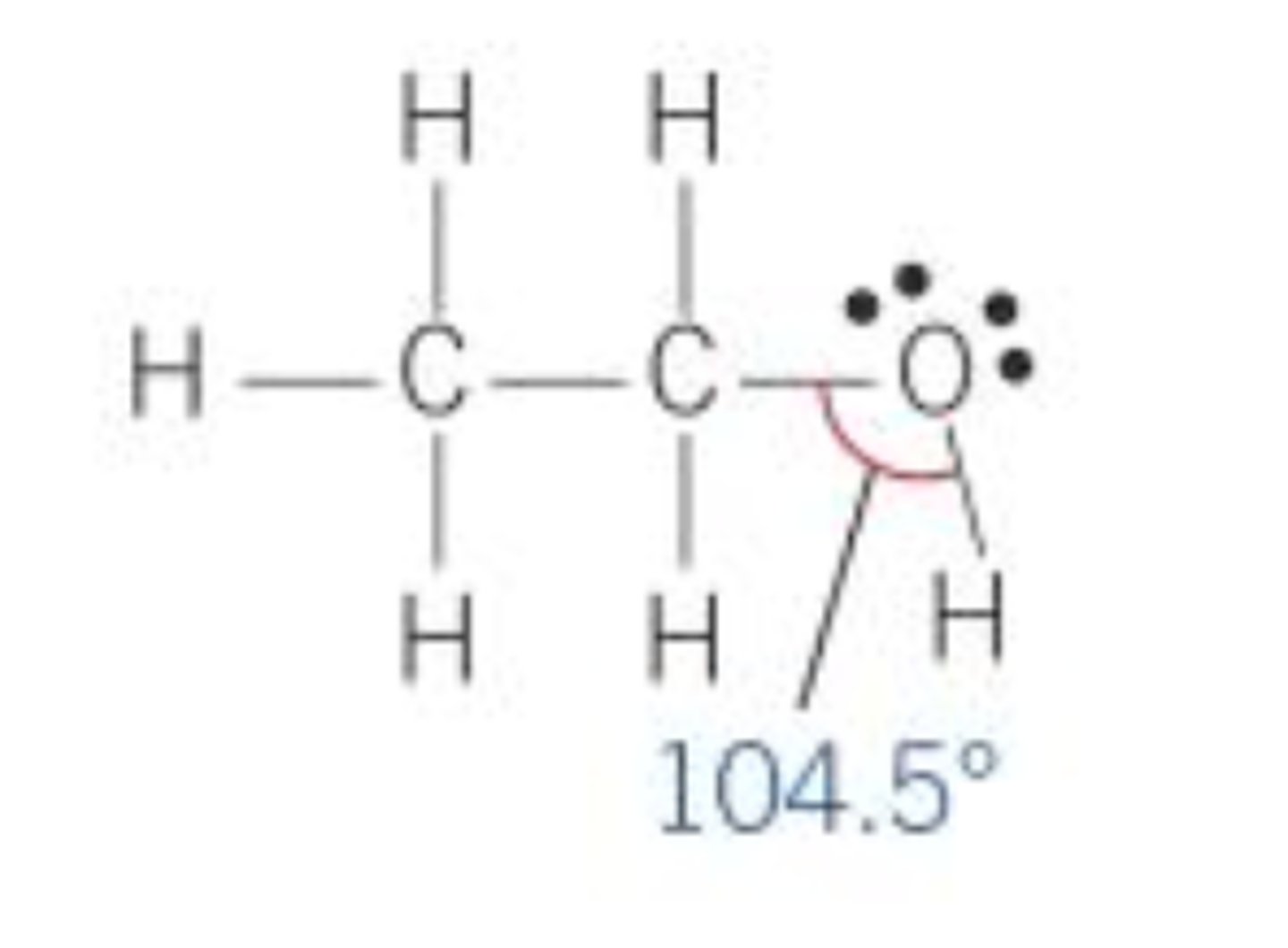
Shape of alcohols
C-O-H angle 104.5 since there are two lone pairs
Why are shorter hydrocarbon chains soluble in water but longer hydrocarbon chains are not
The OH- group of alcohols can hydrogen bond to water molecules, but the non polar hydrogen chain cannot
Shorter chains - soluble in water as the hydrogen bonding predominates
Longer chains - insoluble as the non-polar hydrocarbon chain predominates
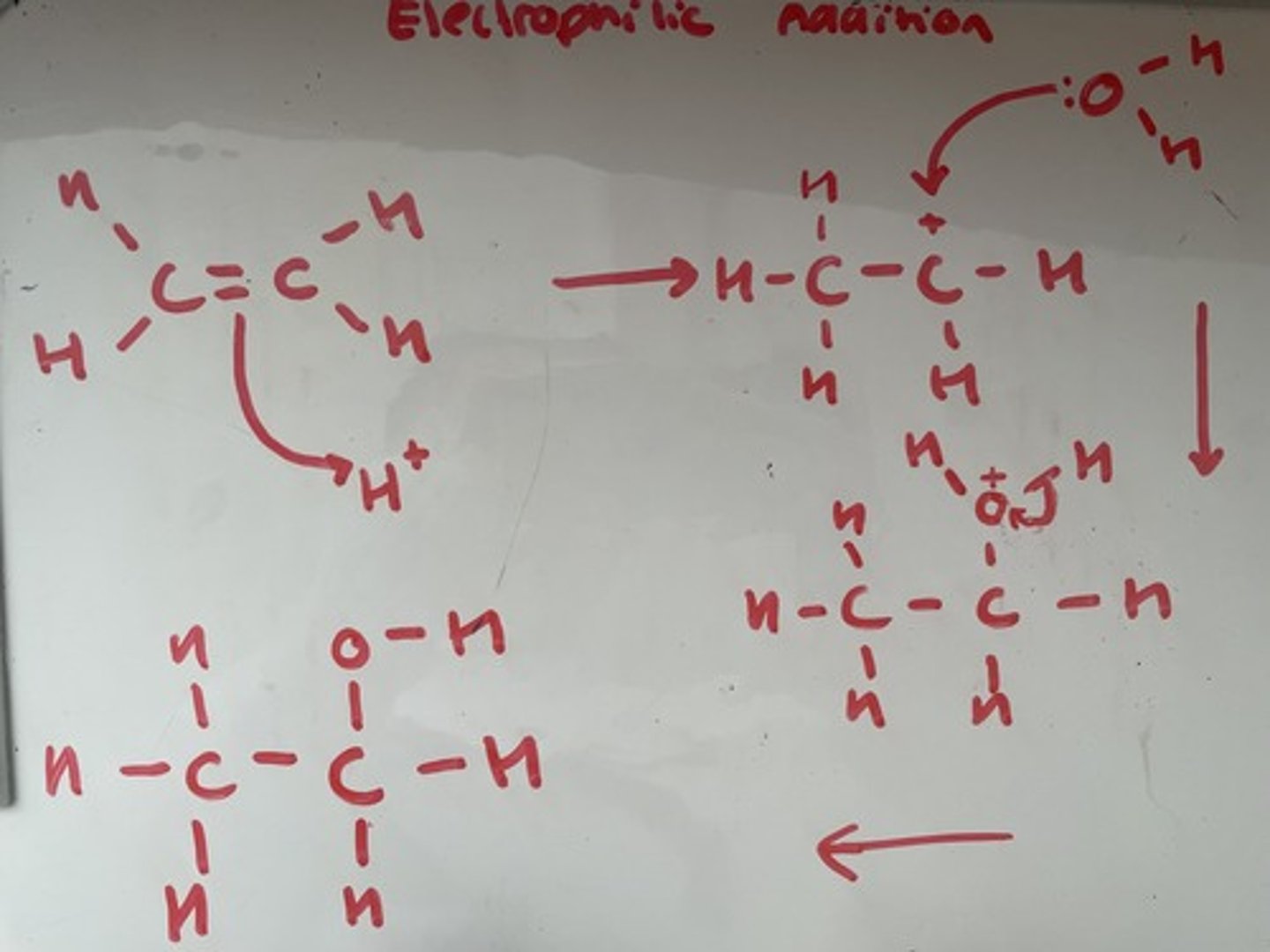
Outline the mechanism for the formation of an alcohol by the reaction of an alkene with steam in the presence of an acid catalyst, use ethene as an example. State the conditions for this reaction
H3PO4 catalyst, high temp (300 degrees) pressure 60atm
a) Dehydration of alcohols
b) How can you make ethene from ethanol
a) CNH2N+1OH ---> CNH2N + H20
You can make alkenes by eliminating water from alcohols
b) Heat ethanol with a H2S04/H3PO4 catalyst
The mechanism for this reaction is the opposite of the mechanism for the formation of an alcohol
The positively charged oxygen pulls electrons away from the carbon, h2o molecule leaves
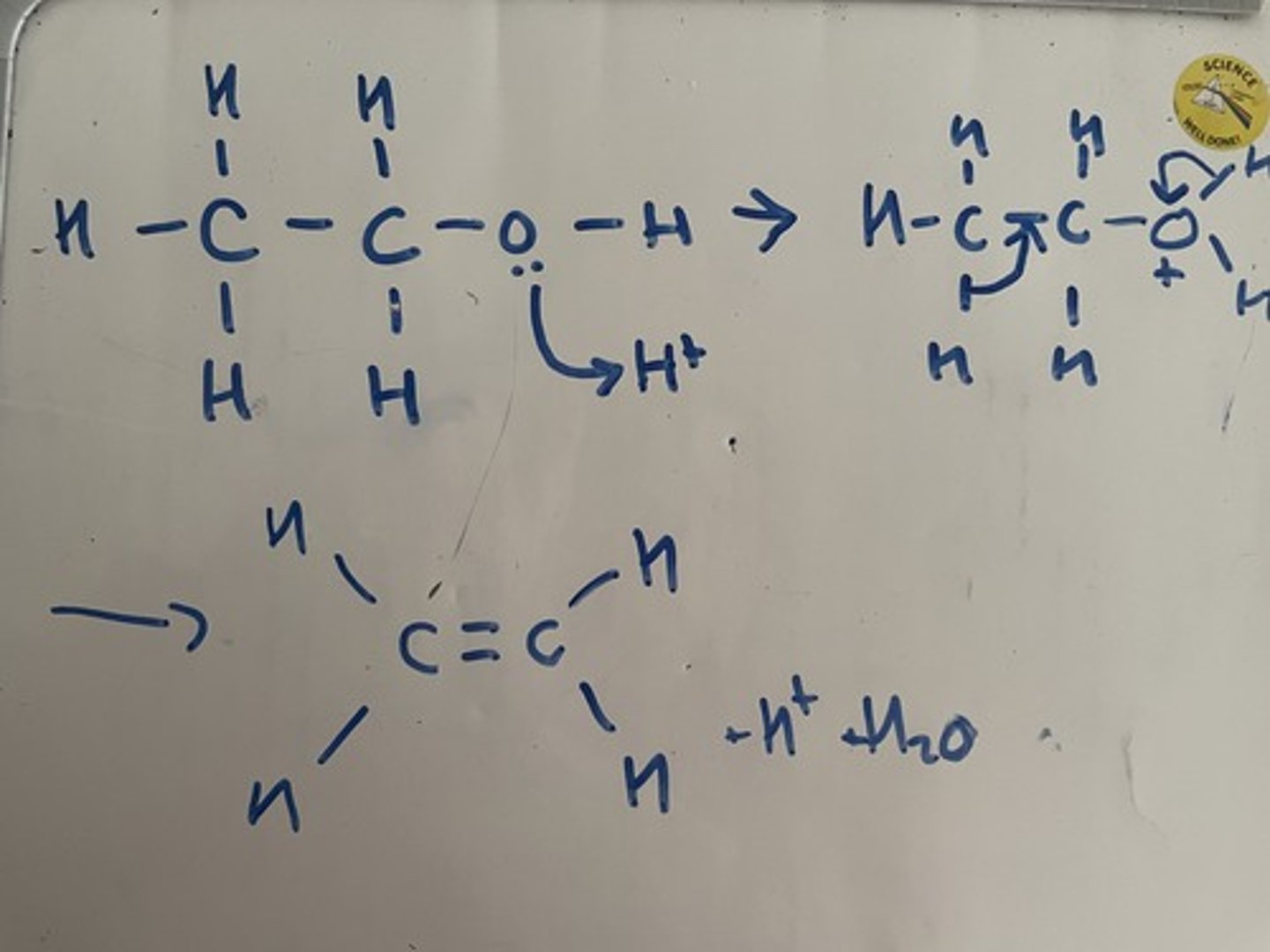
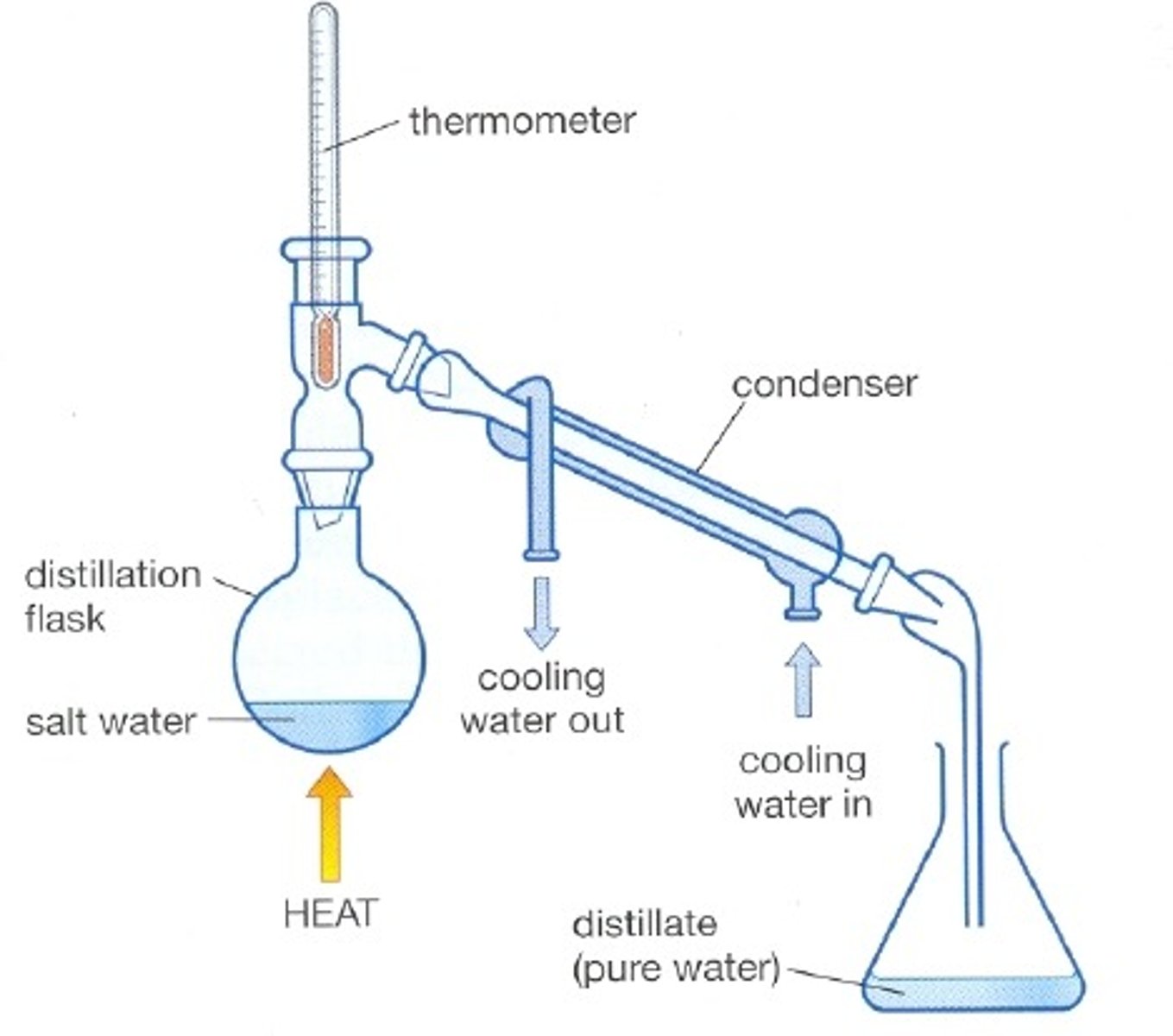
Method to get a pure alkene initially produced from a dehydration reaction, stage 1
Distillation - uses the fact that different chemicals have different boiling points to separate them
For example here's how to produce cyclohexene from cyclohexanol
STAGE ONE: distillation
1) Add conc H2SO4 and H3PO4 to a flask containing cyclohexanol. Mix.
2) Connect flask to rest of distillation apparatus, including thermometer condenser and cooled collection flask
3) Gently heat the mixture to the boiling point of cyclohexene w water bath (chemicals w boiling points lower will evaporate and rise out of flask to condenser)
4) At condensor cooler temps turn the gases back into liquids
5) Liquid product collected
Method to get a pure alkene initially produced from a dehydration reaction, stage 2
Seperation phase - product collected after first stage will still have some impurities
1) Transfer product mixture to separating funnel, add water to dissolve solid impurities --> creates an aqueous solution
2) Allow mixture to separate into layers, drain off aq layer
Method to get a pure alkene initially produced from a dehydration reaction, stage 3
Purification phase
1) Drain off impure cyclohexene into flask
2) Add CaCl2 (drying agent) to flask
3) Distill one more time and the product produced at cyclohexene boiling point will be the pure mixture itself
Industrial production of ethanol by fermentation
Fermentation is an exothermic process
Conditions: 30-40 degrees C, anaerobic conditions
Yeast produces enzymes which concert glucose into ethanol and carbon dioxide
C6H12O6 ---> 2C2H5OH + 2CO2
Fermentation v Hydration of ethene to produce ethanol
Fermentation:
-slow
-impure, needs further processing
-sugars are a renewable energy source
-batch process, cheap but high labour costs
-produces CO2
Hydration of ethene:
-fast
-pure
-ethene from crude oil, finite resource
-continuous process, expensive equipment needed, but low labour costs
-Pollution of hydrocarbons escape
What is meant by a biofuel?
Fuel that is made from biological material that has recently died such as sugars from sugarcane can be fermented to produce ethanol
Ethanol from fermentation is a biofuel
Advantages and disadvantages of biofuels
Advantages:
-Renewable energy resource
-Produces CO2 when burnt but burning a biofuel only releases the same amount of CO2 that the crop plant took in as it was growing, carbon neutral
Disadvantages:
-Occupies land that could have been used to grow food, unable to feed everyone (food v fuel debate)
-Deforestation to create land for biofuels
-Fertilisers used to increase biofuel crop production, pollute waterways, some fertilised produce NOX
-Current car engine would be unable to run on fuels with high concentrations of ethanol
Equations that prove biofuels are carbon neutral
1) Photosynthesis of plants
6CO2 + 6H2O ---> C6H12O6 + 6O2
2) Fermentation, glucose into ethanol
C6H12O6 ---> 2C2H5OH + 2CO2
3) Ethanol is burned
2C2H5OH + 602 ---> 4CO2 + 6H20
If you combine the three equations, exactly 6 mols of CO2 are taken in and also given out
Why might biofuels not be considered carbon neutral
-Fossil fuels will be needed to be burned to power the machinery used to make fertilisers for the crops and machinery used to harvest the crops
-Refining/transporting bioethanol uses energy
Can tertiary alcohols be
a) oxidised
b) dehydrated
a) no
b) yes
What are primary and secondary alcohols oxidised to
Primary - to aldehydes then further oxidised to carboxylic acids
Secondary - to ketones
How can we maximise our yield when oxidising an alcohol through distillation
Vapours are truly condensed, place boiling tube holding product in an ice bath
Method of oxidising an alcohol to make a carboxylic acid straight away
Reflux
a) Ethanol oxidised into ethanal equation
b) Ethanol oxidised into ethanoic acid equation
a) CH3CH2OH + [o] ---> CH3CHO + H2O
b) CH3CH2OH + 2[o] ---> CH3COOH + H2O
Ketone functional group
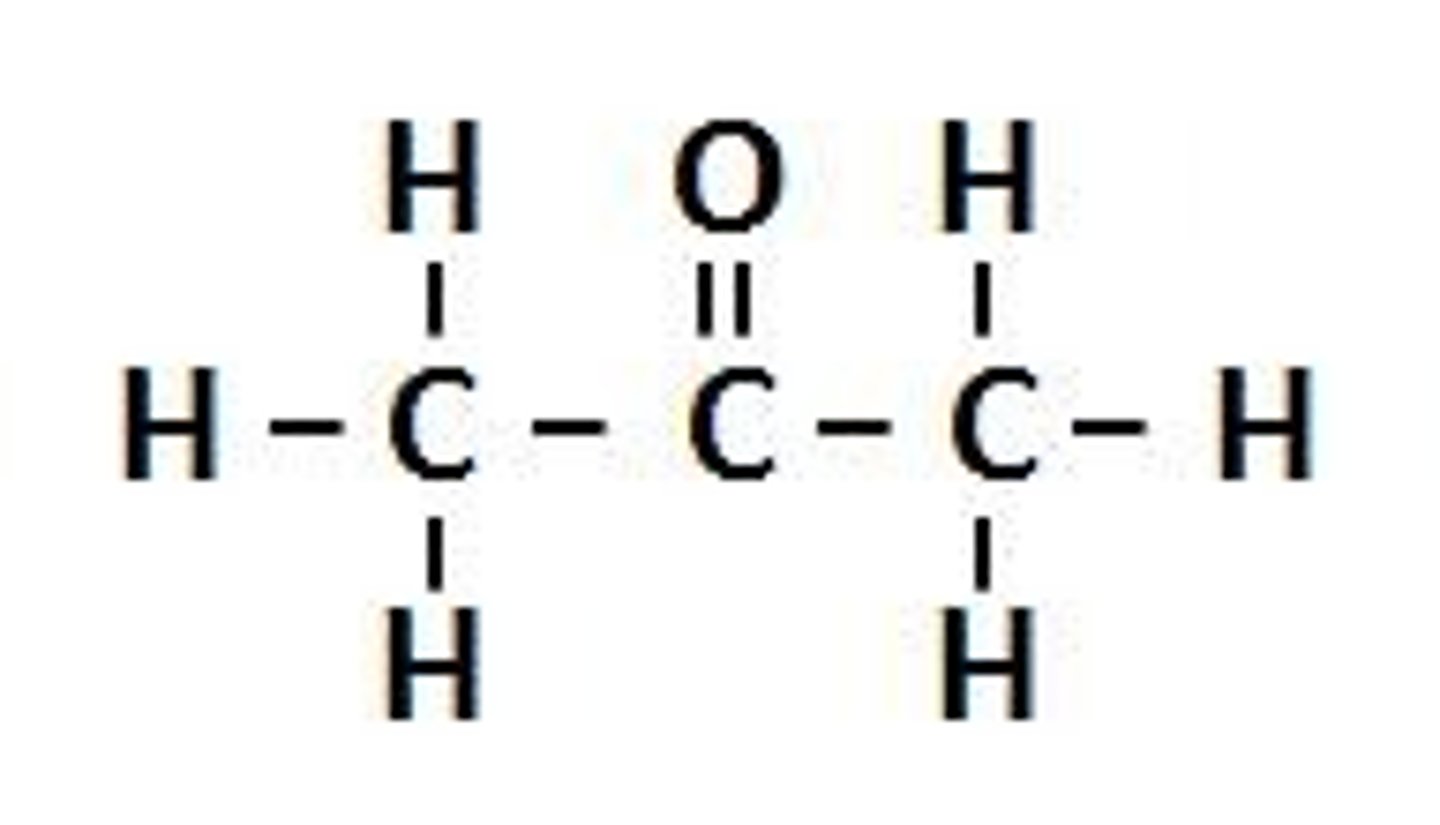
Aldehyde functional group
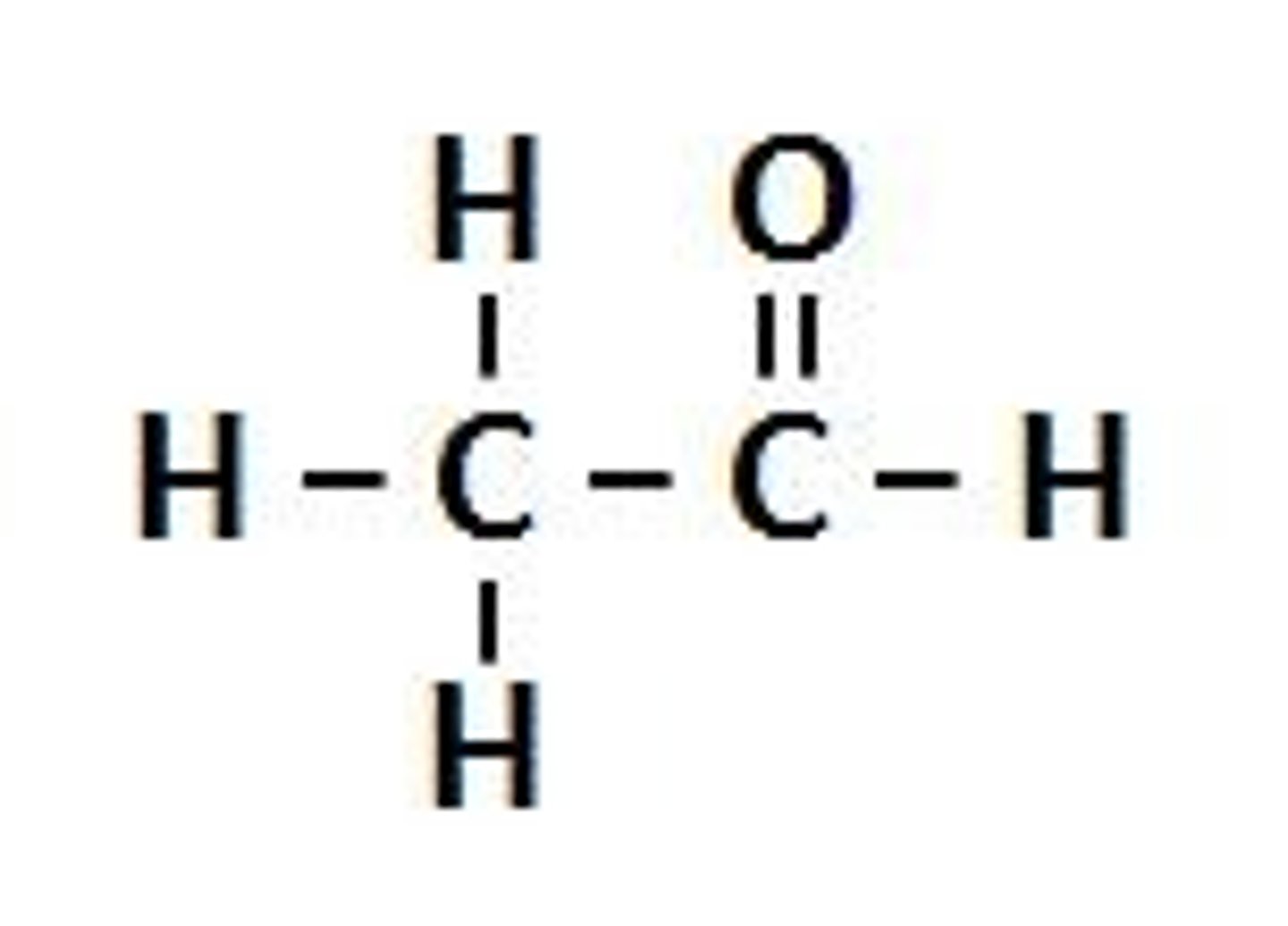
Propan-2-ol oxidised to a ketone
CH3CHOHCH3 + [o] ---> CH3COCH3 + H2O
What determines whether a molecule can be oxidised or not
Carbon to which the oxygen is bonded to must have a removable hydrogen, eg propanone cannot be oxidised further
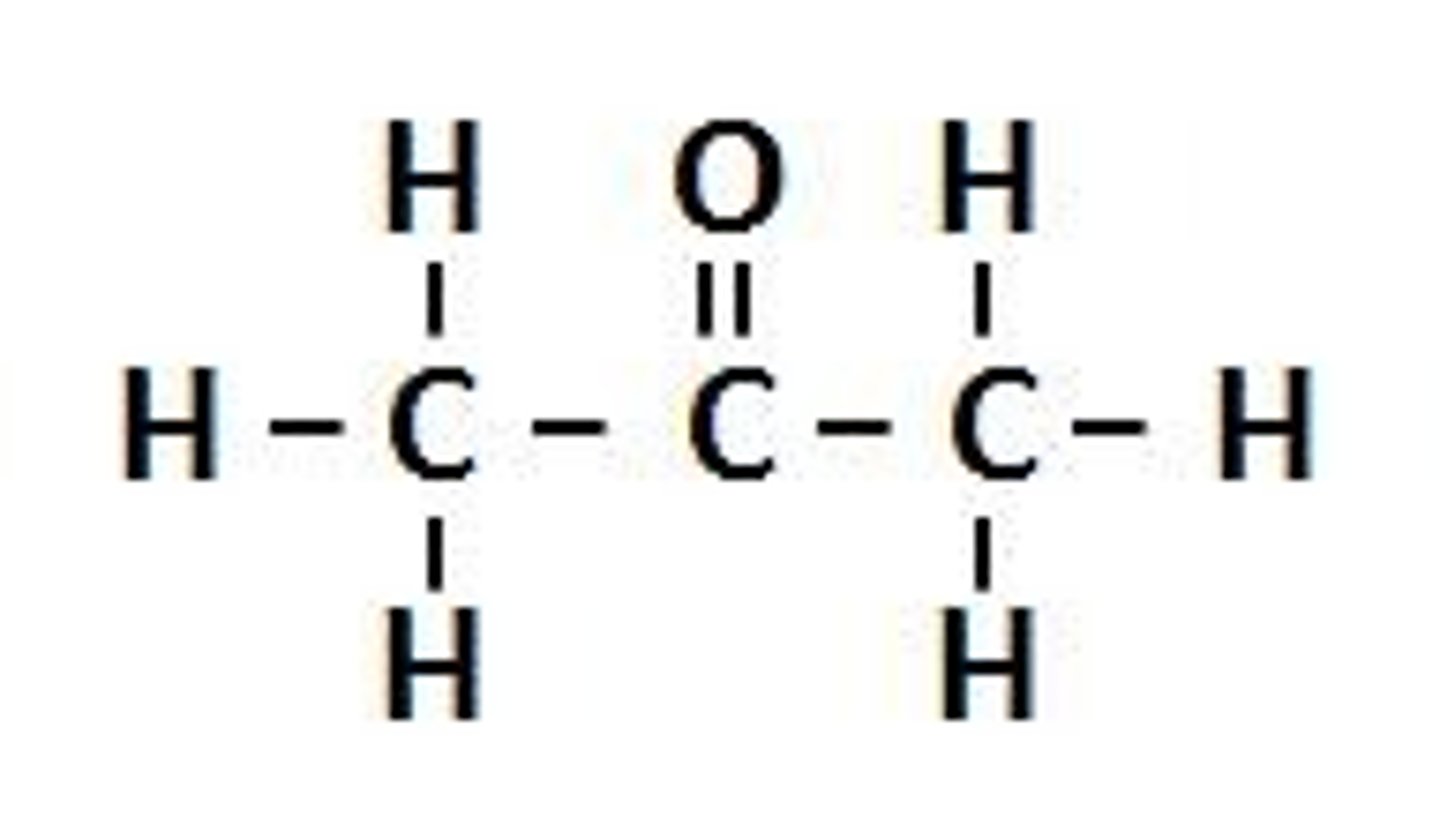
Test to distinguish propanoic acid from propan-1-ol
Add sodium hydrogen carbonate to both
Observation with propanoic acid: effervescence
Observation with propane-1-ol: no visible change
Show how combining the equations from these two methods can lead to the 1:2 mol ratio of carbon monoxide to hydrogen required for this synthesis of methanol (2)
Method 1 CH4 + H2O → 2CO + 3H2
Method 2 CH4 + CO2 → 2CO + 2H2
A method which shows (see below) OR states in words that two times the first equation + the second equation gives the correct ratio
Vegetable oils, which contain unsaturated compounds, are used to make margarine. Identify a catalyst and a reagent for converting a vegetable oil into margarine
Catalyst: Nickel/platinum/palladium
Reagent: Hydrogen/h2
State what is meant by the term hydration (1)
Addition of water/steam
Give a suitable reagent and reaction conditions for the oxidation of ethanol to form the carboxylic acid as the major product
Potassium dichromate
H2SO4
Most of the ethene used by industry is produced when ethane is heated to 900°C in the absence of air. Write an equation for this reaction
C2H6 →C2H4 +H2
Test for primary, secondary and tertiary alcohols
Primary - potassium dichromate turns it from orange to green
Secondary - potassium dichromate turns from orange to green
Tertiary - potassium dichromate remains orange
Test and observation to distinguish between primary and secondary alcohols
Primary are aldehydes/carboxylic acid
Secondary are ketones
Test for aldehyde
Tollens reagent ---> silver mirror
Feelings solution ---> red precipitate
In a case of a positive test an aldehyde, hence a primary alcohol is present.
Ketones (secondary) would not respond to these tests
Test to distinguish between silver nitrate and aqueous sodium nitrate
Reagent: any soluble chloride including HCl
Observation with silver nitrate: white precipitate
Observation with sodium nitrate: no visible change
Can use sodium iodide/bromide
Some alcohols can be oxidised by an acidified solution of potassium dichromate(Vl). Aldehydes can be oxidised by Tollens' reagent or by Fehling's solution.
An unknown pure liquid A contains only a single alcohol.Outline a simple procedure to allow you to determine whether A is a primary, a secondary or a tertiary alcohol
-Mix the alcohol with warm potassium dichromate to test for the presence of tertiary alcohol, there will be no visible change
-Distillation of initial product needed for test of primary/secondary
-Tollens reagent will result in silver mirror of aldehyde (primary alcohol present)
Write an equation for the oxidation of butane-1,4-diol into butanedioic acid
HOCH2CH2CH2CH2OH + 4[O] HOOCCH2CH2COOH + 2H2O
The energy stored in fuels can be compared using energy density values measure in kJdm-3
Calculate the energy density of butan-1-ol
Enthalpy of combustion of butan-1-ol = -2676 kJmol-1
Density of butan-1-ol = 0.810kg moldm-3
Mr of butan-1-ol
Amount of butan-1-ol in 1dm3 = 810/74 = 10.95
10.85 x -2676 = 29300
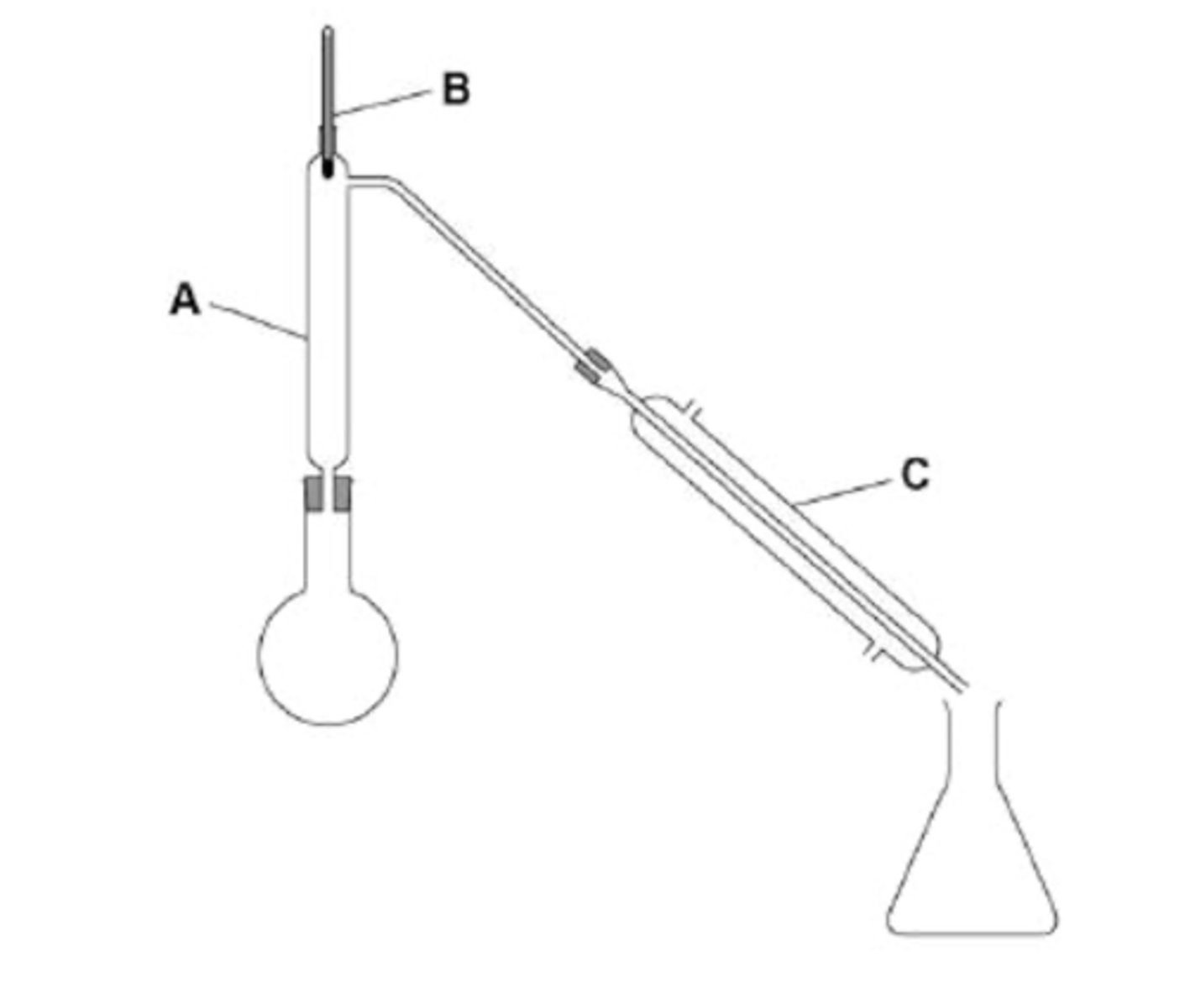
The students then set up the apparatus shown in the diagram below and placed the aqueous mixture in the round bottomed flask.
Describe how the students would use this apparatus to collect a sample of ethanol. Include in your answer the functions of the parts of the apparatus labelled A, B and C.
Stage 1 - turn on the water, heat the flask with a bunsen burner. This causes water and ethanol vapours to be produced
Stage 2 - Vapours pass up the fractionating column A. Water and ethanol are separated in column A
Water condenses back into the flask in column A
Stage 3 - observe the thermometer at B to keep the temperature at or below the boiling point of ethanol
Only ethanol vapour passes into the condenser.
Use the condenser at part C to cool vapours and condense the ethanol back int liquid