Topic 12:Intermolecular Forces
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
IMF
Non covalent interaction between atoms, ions, molecules that determine macroscopic properties (boiling point, viscosity solubility)
How do solids, liquids, and gases differ in IMFs, distance and motion
Solids: strong IMF fixed shape and volume, limited motion
Liquid: medium IMF, fixed volume but shale can vary. Molecules slide around
Gases: weakest IMF, variable shape and volume, molecules move freely
Normal boiling point
Temperature at which a liquid becomes a gas at 1 atm pressure
Relationship of boiling point and IMF
Stronger IMF= higher boiling point (more energy needed to break bonds)
Types of IMF
Ion-ion
Dipole dipole
Hydrogen bonds
London dispersion forces
Ion-dipole interactions
What are ion - ion interactions
Attraction between FULLY charged ions (governed by Coulomb’s law)
Where do ion-ion interactions occur
Ionic compounds that from crystal lattices of alternating cations or anions
Lattice energy
Energy required to separate one mole of an ionic solid into gas ions (pretty much measures ionic bond strength)
2 factors that determine lattice energy
Charge magnitude: greater charges = stronger attraction
Ion size: smaller ions = shorter distance and stronger lattice energy
How do ion size and charge vary across the periodic table
Down a group of= ion size increasing
Across a group of= cation charge increases, size decreases
Anion size changes little overall
How does lattice energy explain melting or boiling points?
Higher lattice energy causes stronger ionic bonds and higher melting boiling points
mgO > NaCl because Mg2+ and O 2- have higher charges
Dipole dipole interactions
Attraction between molecules with permanent partial charges (polar molecules)
Give an example of dipole dipole effects on boiling points
CH4 (non polar, BP -161*C) vs CH3Cl (polar, BP = -24*C)
the polar molecule has stronger IMFs and higher boiling point
London dispersion forces
Weakest attraction formed by electron fluctuations, present in all compounds
What facets the strength of dispersion forces
molecular size and mass (more electrons = more polarizability)
Molecular shape (longer, less branched molecules = more surface contact and stronger dispersion)
Two examples of dispersion force trends
boiling point increases with molecular mass in noble gases or alkanes
Straight chain alkanes have higher boiling points than branched isomers
Hydrogen bond
Strong dipole dipole interaction between a hydrogen afom that is covalentlh. Landed to N, O, or F( the donor) and a lone pair on another electronegative atom (the acceptor)
Define hydrogen bond donor vs acceptor
Donor: atom where H is covalently bonded (N-H)
Acceptor: atom with lone pair accepting the H bond (N, O, F)
Difference between intermolecular and intra molecular hydrogen bonds
Intermolecular: between different molecules
Intramolecular: within the same molecule
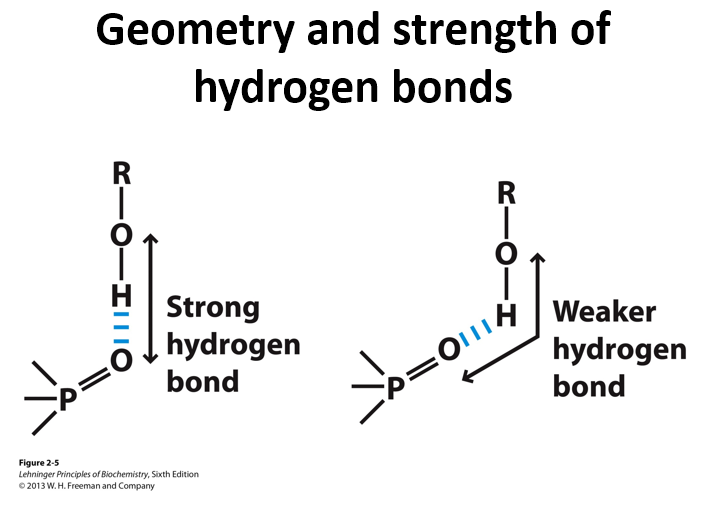
Why are hydrogen bonds directional
they align linearly between the donors H and the acceptors lone pair orbital which gives the most overlap
Give examples of substances with hydrogen bonding
Water, HF, NH3, DNA base pairs, Kevlar fibers
How do hydrogen bonds explain why ice floats
hydrogen bonds in ice make hexagon lattices which lower the density of the ice compared to water
What are ion dipole interactions
Attractions between a charged ion and polar molecule (NA+ and H2O)
How do ion dipole interactions cause ionic compounds to dissolve
Polar water molecules surround ions, stabilize them and break ionic lattices
What is a sphere of hydration
A shell of oriented water molecules surrounding a dissolved ion, reducing electrostatic attraction between ions
Solvent vs solute vs solubility
Solvent : component in greatest amount
Solute: component dissolved in solvent
Solubility: maximum solute that dissolves in a given solvent
How is solubility determined by IMFs
Solubility increases when solute/solvent IMFs are similar in strength and type to those within the solute and solvent
Miscible
Two liquids that mix in all proportions without separating into layers
Like dissolves like
Polar solvents dissolve polar/ionic solutes, non polar solvents dissolve non polar solutes because similar IMFs are compatible
Hydrophobic vs hydrophilic
Hydrophobic: repels water
Hydrophilic attracts water
How do you identify the main IMF in a compound?
Examine molecular polarity, presence of H in N/O/F bonds, and ion presence
What is viscosity
Resistance of a liquid to flow or deformation, a measure internal friction between molecules
How does IMF strengthen viscosity
Stronger IMF = higher viscosity because it has a higher bond and can hold together better
Surface tension
The resistance of a liquid surface to external force due to cohesive IMFs
How does IMF strength affect surface tension
Stronger IMF = higher surface tension
Rank IMF from strongest to weakest
Ion-ion, ion dipole, hydrogen bonds, dipole dipole, London dispersion