Lecture 4: Motor Disorders
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
motor control
-cortex issues commands based on integration of sensory inputs
-a copy is sent to basal ganglia and cerebellum
-these feedback to cortex via thalamus
-command that reaches lower motor neurons is continually modulated by basal ganglia and cerebellum
motor cortex
-issues descending motor commands for muscle activation
-regulates activity levels in spinal cord circuits
-where the upper motor neuron begins
motor cortex damage symptoms
-impaired voluntary movement
-poor high level coordination
-weakness of voluntary movement
-upper motor neuron syndrome
cerebral palsy (damage to motor cortex)
-result of damage to motor control structures of the brain
-injury often occurs pre or peri natally
-stiffness and weakness of muscles
-poor coordination
-affects upper motor neurons
-varied effects on different people
stroke (damage to motor cortex)
-interruption of blood supply to cortex
-upper motor neurons affected
-symptoms depend on extent and location but usually typical of motor cortex damage
cerebral haemorrhage (main cause of stroke)
-often results from an aneurism
-blood is toxic to neural tissue
-if an aneurism can be spotted before rupture can sometimes be treated
cerebral ischaemia (main cause of stroke)
-caused interruption of blood supply to part of the brain due to blockage of a blood vessel
-lack of oxygen/glucose leads to excitotoxicity and neuronal cell death
-can be caused by thrombus or emboli or cardiovascular disease
core region (cerebral ischaemia)
-the region most severely impacted by loss of blood supply
-hard to salvage
penumbra region (cerebral ischaemia)
-around core area where interruption to blood supply may not be total due to overlapping blood supplies
-key for treatment as has not fully lost blood supply and if can remove cause of stroke then potential for good recovery
fine motor control (damage to motor cortex)
-most prominent and widespread symptoms of motor cortex damage relate to fine motor control
-homunculus → there are large representations for these activities and unlikely to be missed by damage, so require coordination across sub-regions
upper motor neuron syndrome
-collection of symptoms that result from damage to UMNS or their pathways
-leads to lack of voluntary control of muscles via lower motor neurons
-lack of regulation of lower motor neurons and spinal reflex circuits
-reflexes become abnormal
babinski reflex (upper motor neuron syndrome)
-stimulate inside of foot arch and move to ball of foot
positive response → toes curl upwards, implies an issue with UMNs
normal response → toes curl downwards
-indicates cortical damage or issues with spinal cord
difficulties separating impact on cognitive and motor function
-people are often perceived to have cognitive impairments but this can simply just be the effect of damage on motor control of speech
-can have nothing wrong cognitively and expression of speech be impaired due to motor issues
conditions implicated in basal ganglia dysfunction
Parkinson’s disease
Huntington’s disease
Tourette’s syndrome
tardive dyskinesia
hemiballismus
→ all linked by impaired selection of appropriate sequences of movements
Parkinson’s disease (basal ganglia)
-involuntary tremor, slowness of movement, rigidity
-difficulty initiating voluntary movements
Huntington’s disease (basal ganglia)
-sudden, jerky, involuntary movements with no purpose
-no weakness, ataxia or sensory deficit
Tourette’s syndrome (basal ganglia)
-sudden repetitive, involuntary movements or utterances
tardive dyskinesia (basal ganglia)
-repetitive, involuntary, purposeless movements
-difficulty in stopping movements
hemiballismus (basal ganglia)
-violent, involuntary movements
-ballistic movements
monogenic Parkinson’s disease
-around 10% of cases occur due to mutation of one or several genes
sporadic Parkinson’s disease
-around 90% of cases don’t seem to be linked to genetic change
-do not know cause of disease
importance of studying monogenic forms of diseases
-makes it easier to unpick the causes and risk factors for these conditions
-have a pathway of change from simple levels (genes) to complicated levels (the condition)
-helps develop treatments for all population with condition and not just monogenic patients
genetic causes of primarily non-genetic diseases
-if a gene mutation is reliably associated with the common clinical phenotype it may tell us about the downstream cellular/molecular events responsible for the condition overall
gene → protein → cell behaviour → CNS pathway → Parkinson’s disease
-can make the puzzle more tractable, gives clues for developing drugs to target affected proteins
-develop gene therapy techniques to alter the faulty genetic messages
-easier to do this in genetic cases than sporadic cases
symptoms of Parkinson’s disease
all underlined by impaired selection of movement
-paucity of spontaneous movement → insufficiency of movement
-bradykinesia → very slow movements
-akinesia → no movements
-increased muscle tone
-resting tremor → pill rolling
-shuffling gait and flexed posture, impaired balance
-mask-like expression
basal ganglia and Parkinson’s disease
-nigrostriatal dopamine neurons degenerate and die
-so don’t get release of dopamine → no excitatory output
-basal ganglia still inhibits excitatory output
-can give drugs that act as building blocks of dopamine (L-dopa) → helps remaining neurons build dopamine to create excitatory output
limits of L-dopa
-PD arises following degeneration of nigrostriatal neurons
-so increasing dopamine availability using drugs stops being effective if there are too few functioning cells left to release dopamine
-doesn’t address non-motor symptoms of PD
non-motor symptoms (Parkinson’s disease)
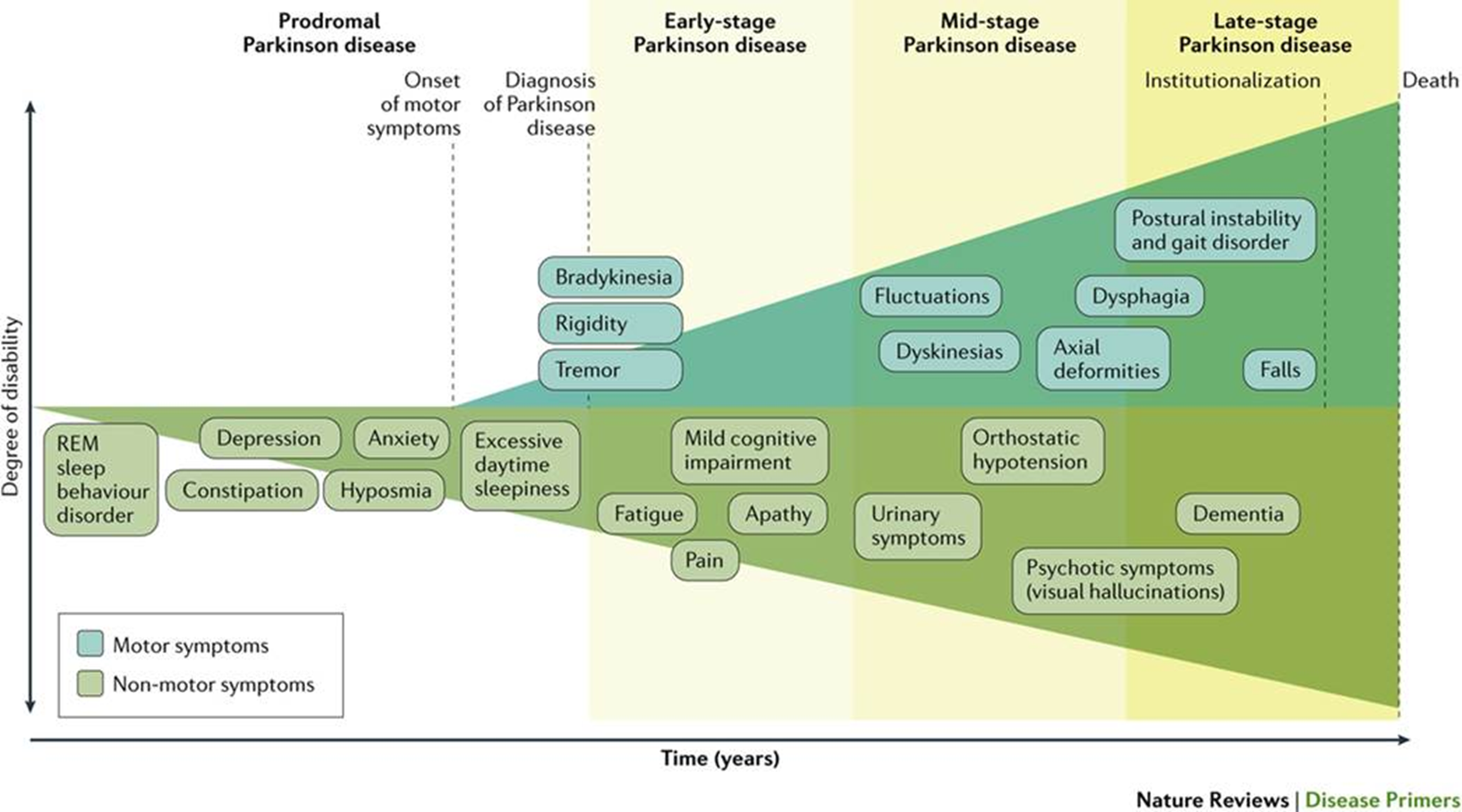
-lots of severe non-motor symptoms associated with PD
-depression, anxiety, regulating sw cycle, pain, dysphagia, hypotension, dementia and cognitive impairment
-underrecognized, underdiagnosed and undertreated as motor symptoms are dominant
deep brain stimulation (Parkinson’s disease)
-electrical stimulation of specific basal ganglia structures to counteract excessive inhibitory output
-blocks and reduced excessive inhibition which allows for return of functions
-allows excitation so people can perform motor programmes
limits of deep brain stimulation
-side effects
-not similarly effective for all
-fails to deal with non-motor symptoms
-confounded by DBS typically being used in later stages
-may make some non-motor symptoms worse
alpha-synuclein (Parkinson’s disease explanation)
-protein
-misfolding and aggregation into Lewy bodies is a hallmark of PD
-impair normal neuronic function
alpha-synuclein and lewy bodies (Parkinson’s disease explanation)
-happens throughout the brain → tends to start in olfactory bulb and spreads as disease progresses
-dopamine neurons in substantia nigra may be particularly vulnerable
-accounts for non-motor symptoms
cerebellum damage
-results in ataxia
-two types of impairment:
disturbances of posture or gait
decomposition of movement
-voluntary movement loses fluidity and appears mechanical, slow and robotic
intention tremor (cerebellum damage)
-when go to move/make action have tremor
-no tremor when resting
dysarthria (cerebellum damage)
-disruption of fine control of speech, slurring
-speech is a fine and controlled process of muscles and cerebellum damage impairs these muscle movements
ataxia (cerebellum damage)
-collection of disorder, unified by their symptoms rather than their causes
-loss of voluntary coordination of muscles → neurological finding in a patient and not a disease
types of ataxia (cerebellum damage)
focussing on types of symptom → cerebellar, sensory, vestibular
focussing on type of cause → acquired, hereditary, late onset cerebellar dysfunction
focussing on more detailed diagnosis
motor neuron disease (MND)
-disease of motor neurons
-leads to degeneration of direct motor control pathway
-execution of motor plan through motor neurons is impacted
-degeneration of motor neurons and muscle wasting
degenerative
progressive
incurable
causes of MND
~10% of cases have genetic component
-90% have environmental, toxic, viral and other factors implicated
types of MND
-ALS is most common subtype of MND
-general distinction of subtypes is related to effects on either upper or lower motor neurons
-ALS affects both types
symptoms of MND
-often altered cognitive function, ability to communicate and affective changes
-complications arising due to impaired respiratory function is often cause of death → affects muscles in chest relating to breathing
treatment and recent research
identifying biomarkers for early diagnosis
identify risk factors
novel neuroprotective drugs
gene therapies
drug to cure and slow progression
clinical and technological interventions to improve and prolong life
SOD1 gene (MND)
-mutations cause genetic form of MND (ALS)
-new drug tofersen prevents SOD1 production
-CSF biomarkers indicated early effects but no benefit at 6 months
-at 12 months there was patient benefit
-SOD1 ALS is rare but major breakthrough in treatment
genetic drug targeting is effective
CSF biomarkers are reliable