Biochemistry - Chapter 9 - Mechanisms and Inhibitors
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Covalent Catalysis
The active site contains a nucleophile that is briefly covalently modified.
General Acid–Base Catalysis
A molecule other than water donates or accepts a proton.
Metal ion Catalysis
Metal ions function in a number of ways, including serving as an electrophilic catalyst.
Catalysis by approximation and orientation
The enzyme brings two substrates together in an orientation that facilitates catalysis.
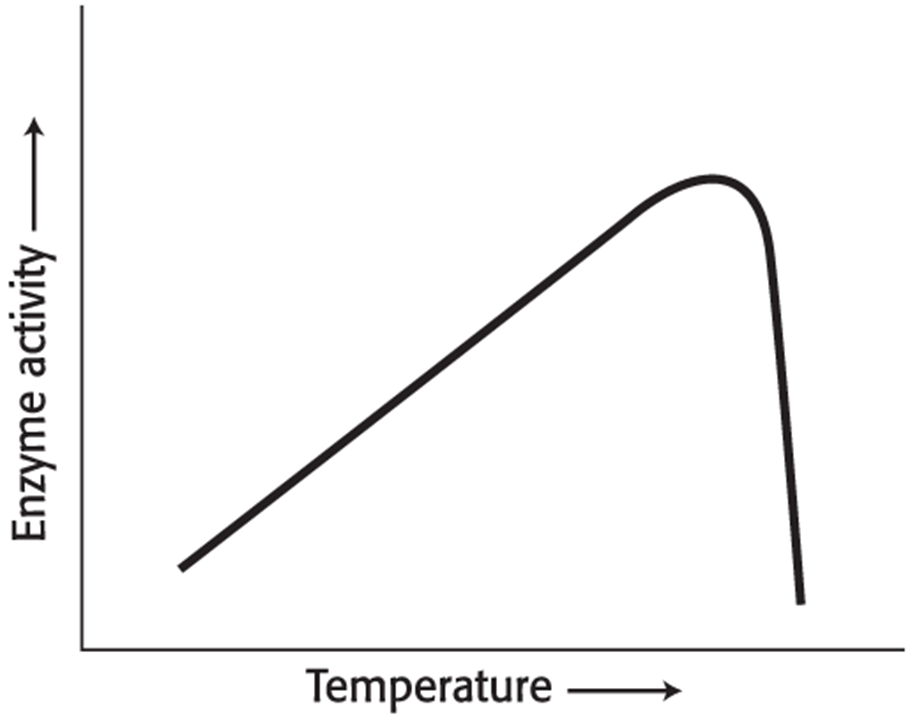
Temperature Enhances the Rate of Enzyme-Catalyzed Reactions
If the pH is lowered (the H+ concentration increases), the -COO- groups will gradually be converted into..
… -COOH groups with a concomitant loss of enzyme activity.
On the other side of the optimum, as the pH is raised (less H+, more OH-), the NH3 + group loses…
…an H+ to OH-, becoming a neutral NH2 group, and the enzyme activity is diminished.
What are the three common types of reversible inhibition?
Competitive inhibition
Uncompetitive inhibition
Noncompetitive inhibition
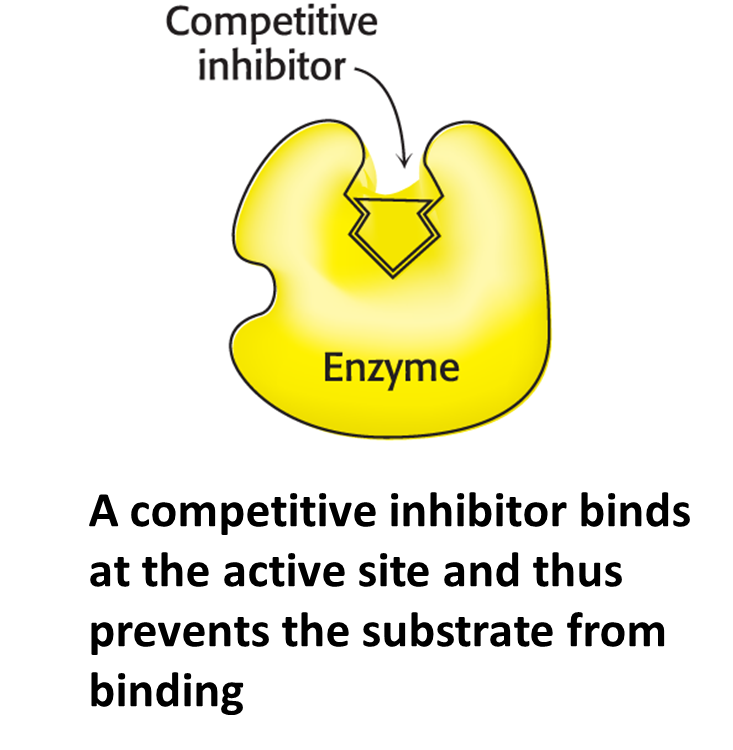
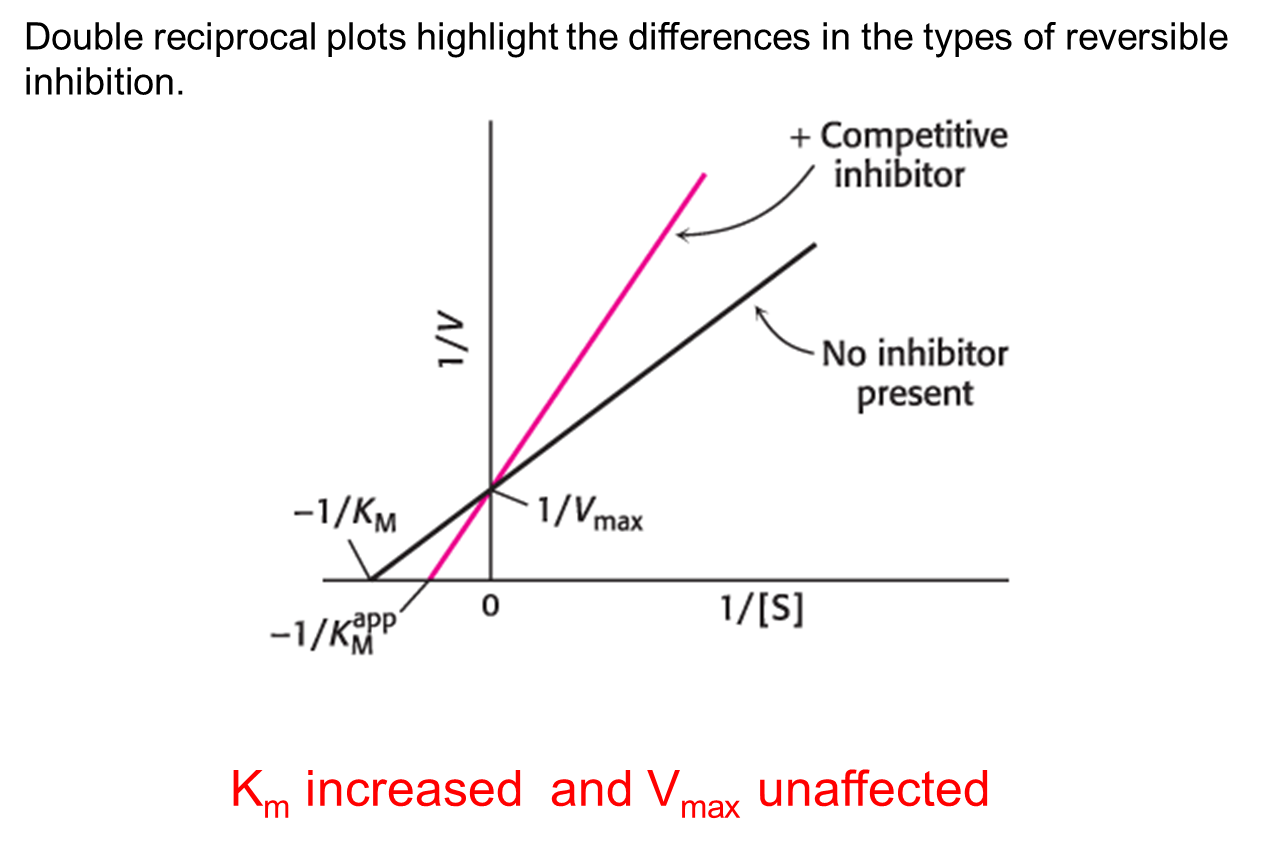
Competitive inhibition
The inhibitor is structurally similar to the substrate and can bind to the active site, preventing the actual substrate from binding.
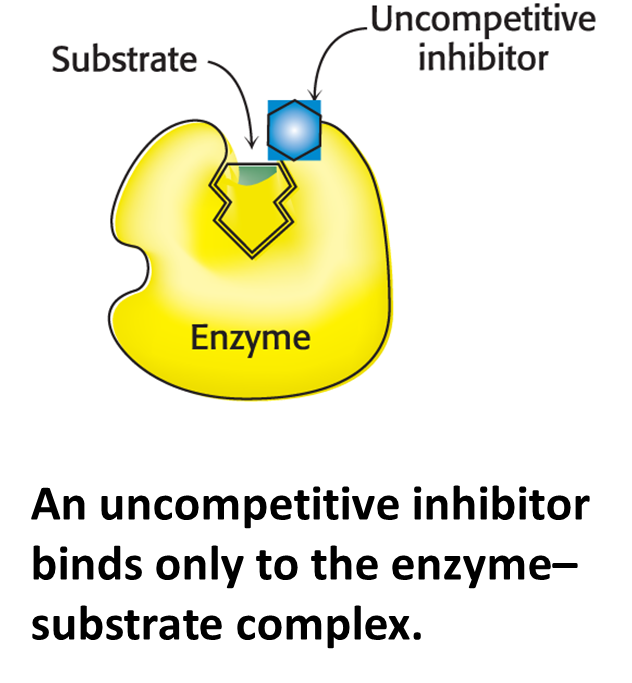
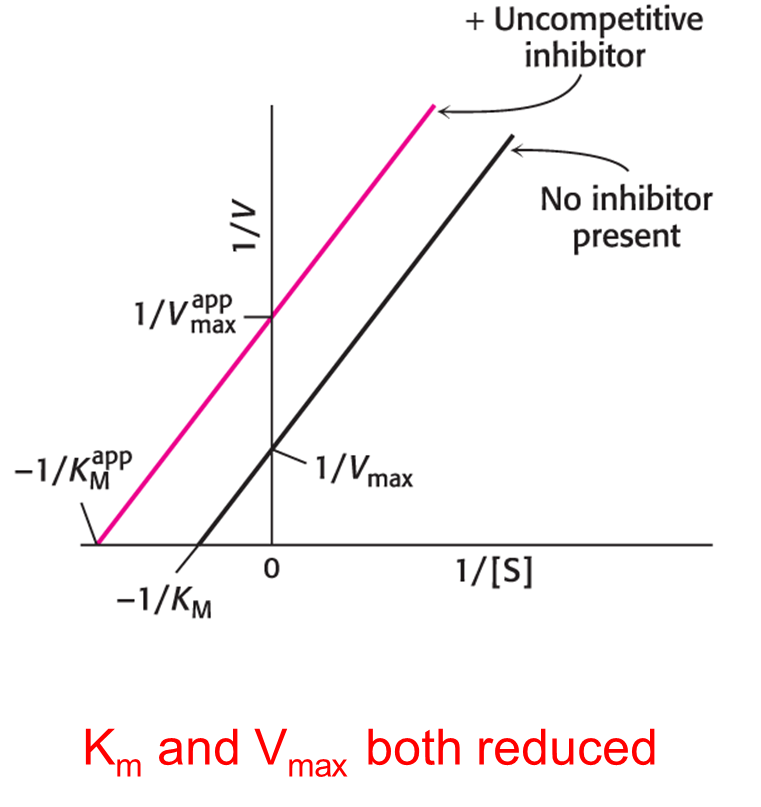
Uncompetitive inhibition
The inhibitor binds only to the enzyme–substrate complex.
Noncompetitive inhibition
The inhibitor binds either the enzyme or enzyme–substrate complex.
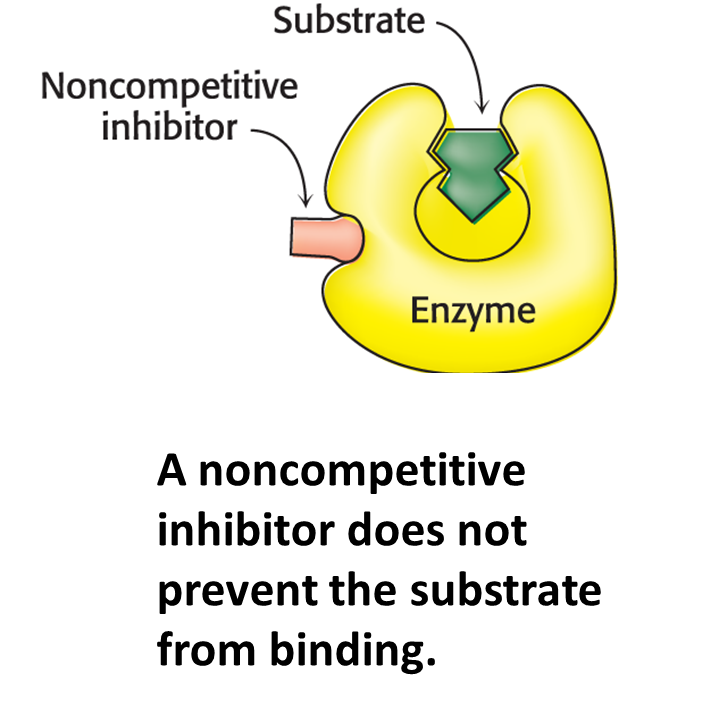
Substrates binds to an enzyme’s to form an…
…enzyme-substrate complex.
What are the differences and similarities of reversible & irreversible inhibitors?
Reversible
Reversible inhibitors bind very loosely to enzymes.
Reversible inhibitors that bind the enzyme ionically and enzyme becomes active when the inhibitor is removed from the environment
Prevent the cell from producing unneeded resources
Irreversible
Irreversible inhibitors bind very tightly to enzymes.
Irreversible inhibitors that bind the enzyme covalently and enzyme remains inactive when the inhibitor is removed from the environment
Many are toxins that interfere with metabolic processes
As the concentration of a competitive inhibitor increases, higher concentrations of substrate are required to attain a particular reaction velocity.
The reaction pathway suggests how sufficiently high concentrations of substrate can completely relieve competitive inhibition.
In uncompetitive inhibition, the enzyme-inhibitor-substrate complex does not form product. Consequently, Vmax is what? Km is what?
Vmax = is lower in the presence of inhibitor
Km = is also lower
Uncompetitive inhibition cannot be overcome by the addition of excess substrate.
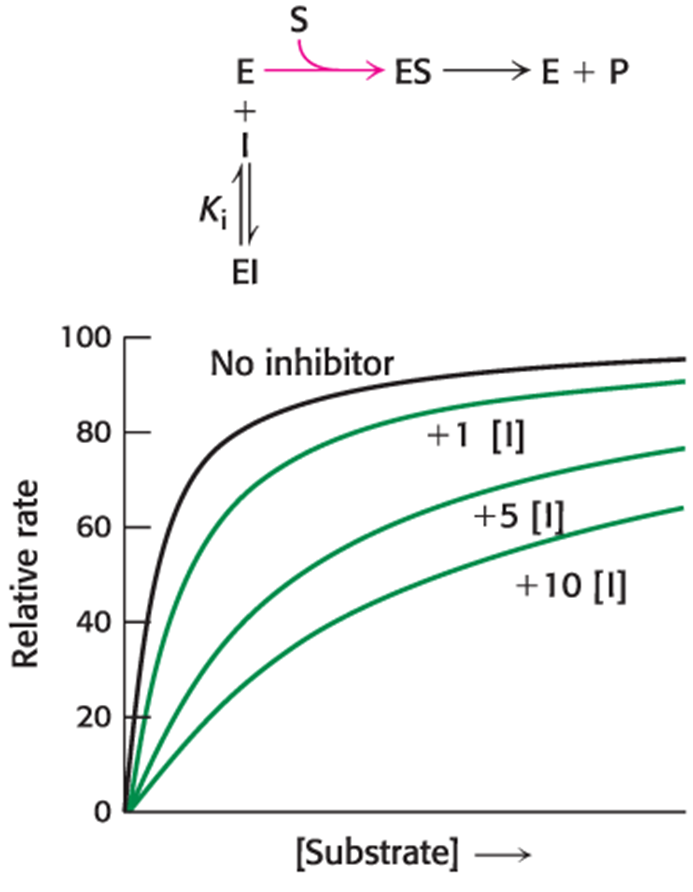
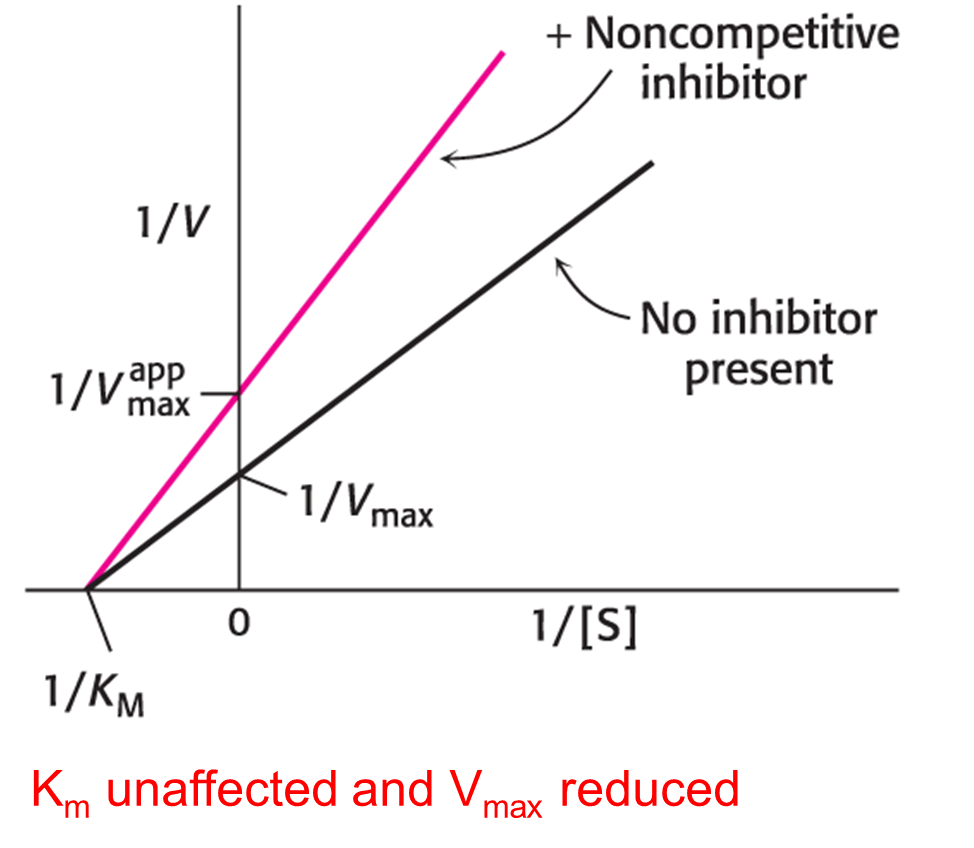
In noncompetitive inhibition, the inhibitor can bind to free enzyme or to the enzyme–substrate complex. In either case, the binding of inhibitor prevents the formation of product. Consequently, Vmax is what? Km is what?
Vmax = is lower in the presence of a noncompetitive inhibitor
Km = is not changed by the presence of a noncompetitive inhibitor.
Noncompetitive inhibition cannot be overcome by increasing substrate concentration.
Double-reciprocal Plot of Competitive Inhibition
Double-reciprocal Plot of Uncompetitive Inhibition
Double-reciprocal Plot of Noncompetitive Inhibition
Chymotrypsin
A proteolytic enzyme secreted by the pancreas that hydrolyzes peptide bonds selectively on the carboxyl side of large hydrophobic amino acids.
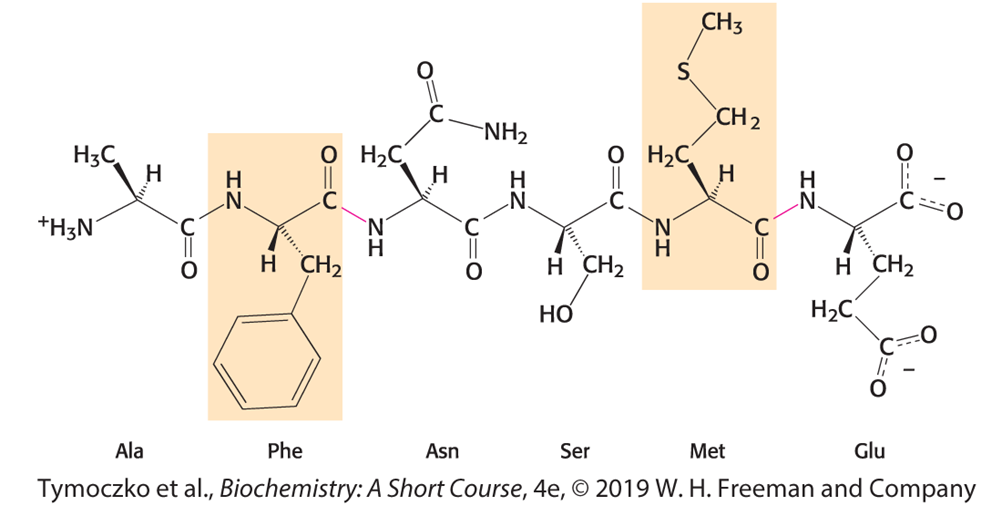
In the process of catalysis, serine 195 becomes
a nucleophile that attacks the carbonyl group of the protein substrate. The group-specific reagent diisopropylphosphofluoridate (DIPF) modifies only serine 195, one of 28 serine residues in chymotrypsin, and inhibits the enzyme.
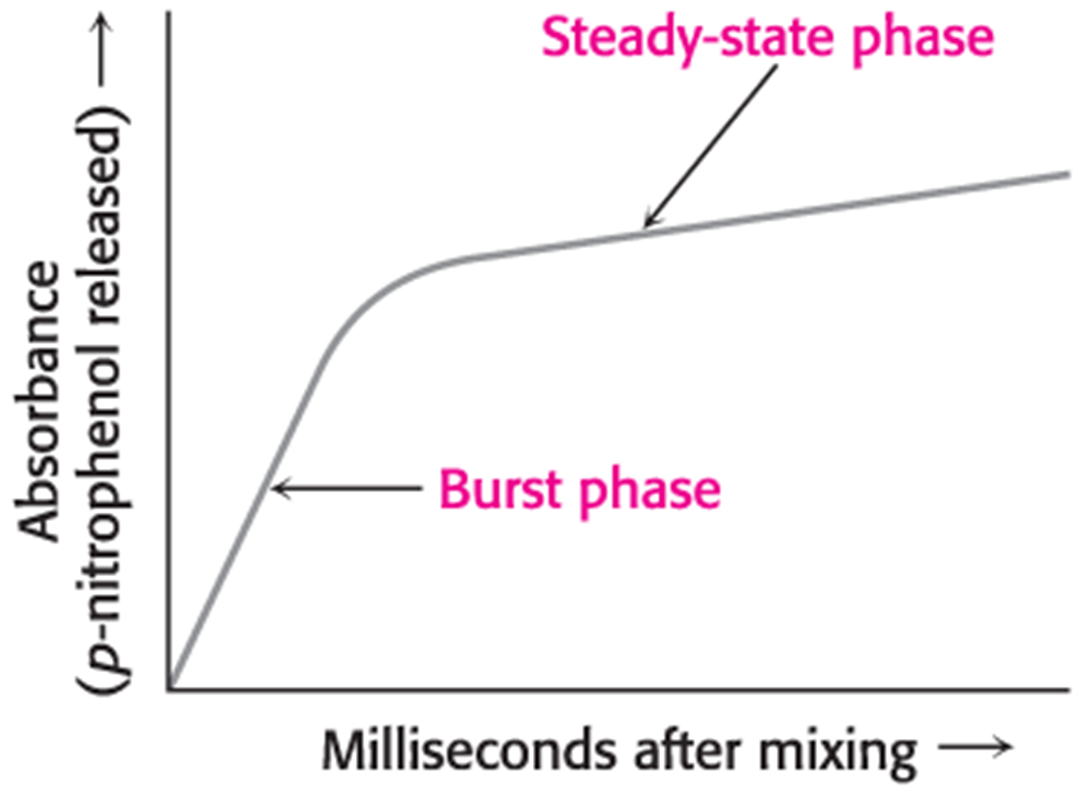
Studies with the chromogenic substrate reveal that catalysis by chymotrypsin occurs in two stages: a rapid step (pre-steady state) and a slower step (steady state).
The steps in catalysis are explained by the rapid formation of an acyl-enzyme intermediate and a slower release of the acyl component to regenerate the free enzyme.