3.1.1 Fundamental particles
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
What principle quantum number (or n) do we refer to each shell in the electron configuration?
1st shell: n=1
2nd shell: n=2
3rd shell: n=3
4th shell: n=4
How do we calculate how much electrons each shell can hold?
2n*2
E.g. 1st shell(1*1=1 → 2X1=2) 2 electrons
2nd shell (2*2=4 → 2X4=8) 8 electrons
What is an orbital?
A region around the nucleus that can hold up to 2 electrons with opposite spins. (Electrons can either have an up spin or down spin) (When we have 2 electrons in the same orbital, these two electrons must have opposite spins)
What is an electron?
A cloud of negative charge. ( the negative charge cloud has the shape if the orbital occupied by the electron)
Each orbital can only hold 2 electrons. There are 4 types of orbitals and there is a different amount present in the electron configuration for each orbital. Name these orbitals and say the amount present?
S: present in all shells (There are 1 s orbitals)(spherical shape)
p:present in all shells apart from the 1st shell. (There are 3 p orbitals) (dumbbell shape)
d:Present in shell 3 and 4 (There are 5 d orbitals)
f:Present in all shell 4 (There are 7 f orbitals)
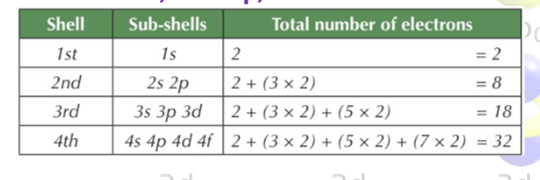
What is a subshell?
All of the orbitals of the same type in the same shell.
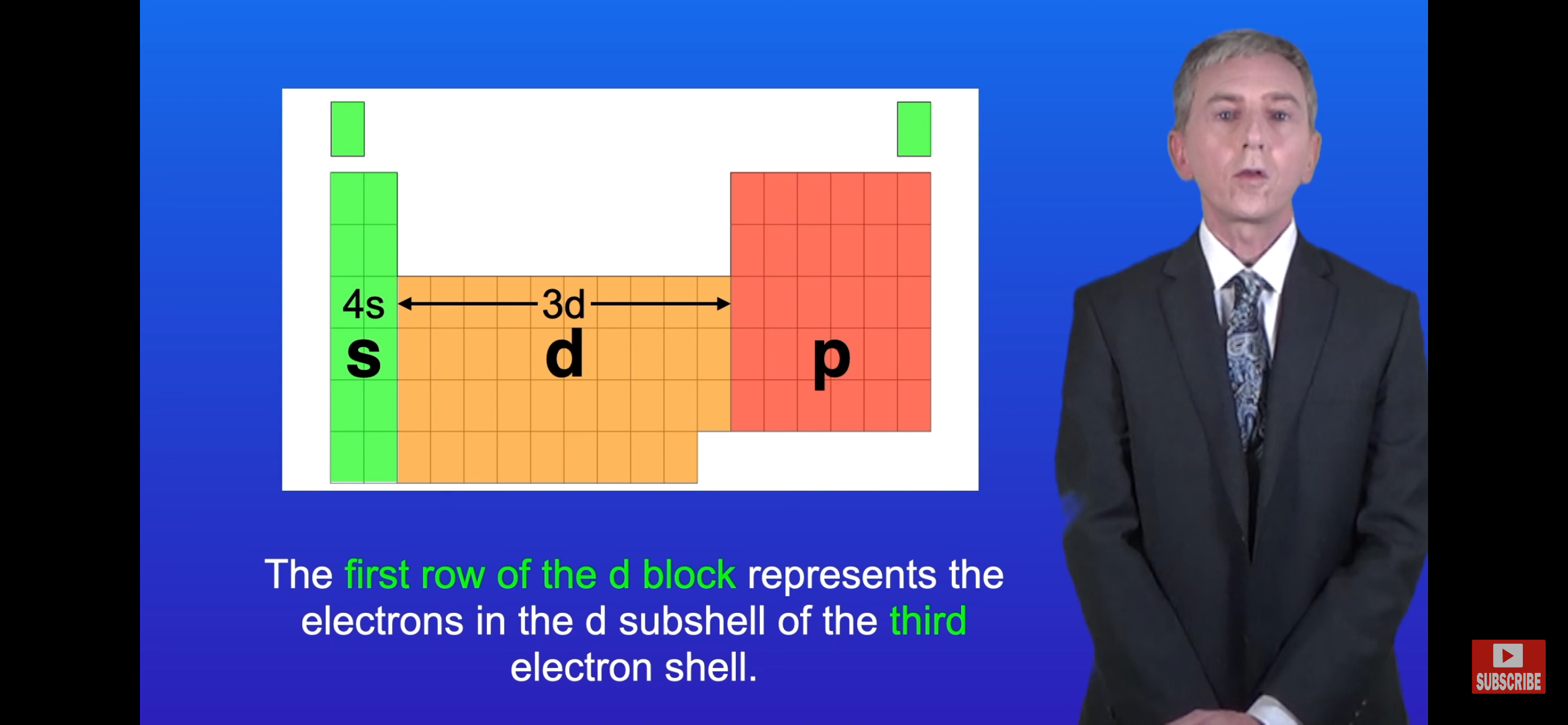
What would the electron configuration of vanadium (Ar=23) be? Why did 4s fill out before 3d?
1s*2 2s*2 2p*6 3s*2 3p*6 3d*3 4s*2
Because sub-shell 3d has a higher energy level than sub-shell 4s

Why do the 4s sub-shells in copper and chromium only have 1 electron even though there are electrons in the 3d sub-shells.
The 3d sub-shell is more stable when it is half-full or completely full so the 4s sub-shell donates its electron to the 3d sub-shell in copper and chromium.
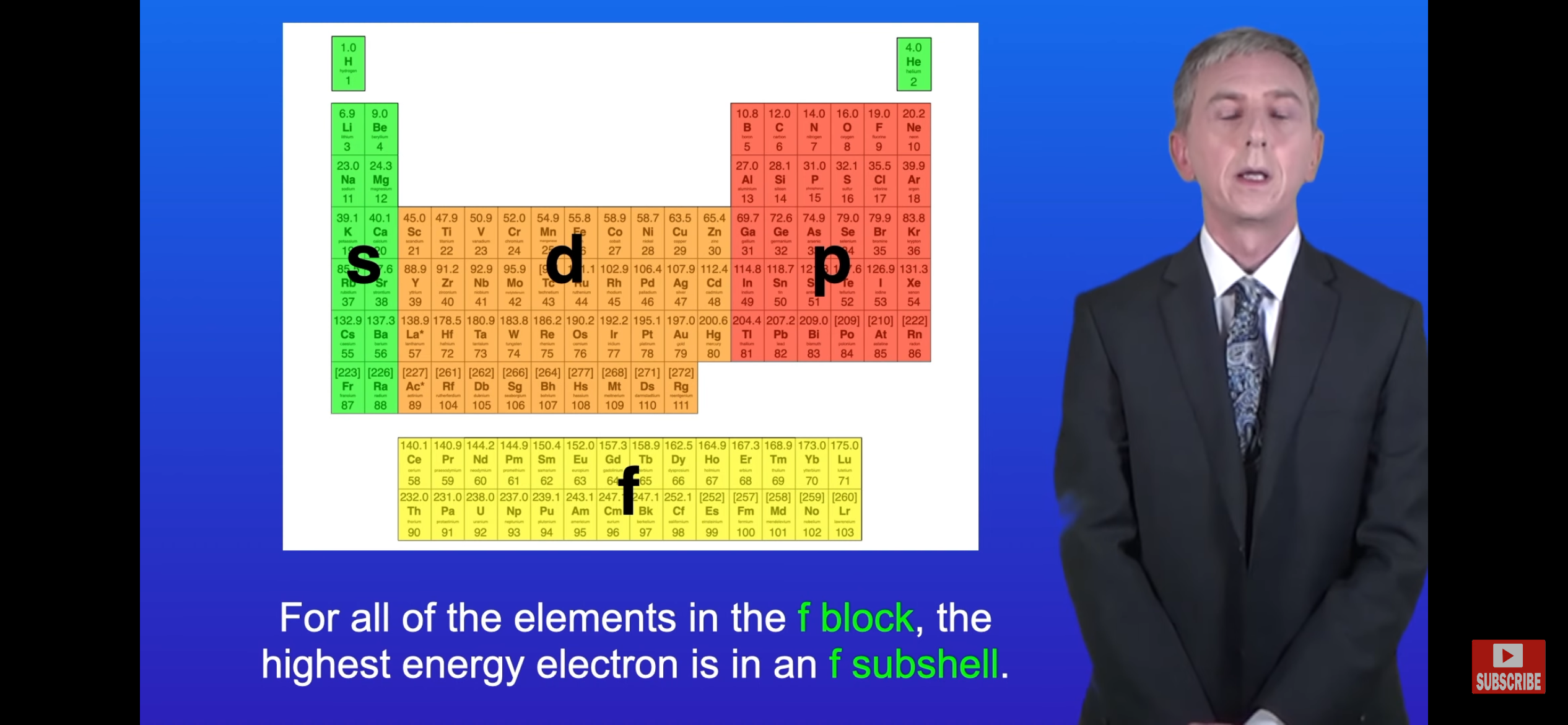
Note- The elements in specific blocks all have their outermost shell in that specific sub-shell.
1.What sub-shell is sodium’s outer electron in?
Note-The rows tells us the specific sub shell that elements have their outermost electron in.
What sub-shell is sodium’s outer electron in?
The s-sub shell. 2. 3s-sub shell
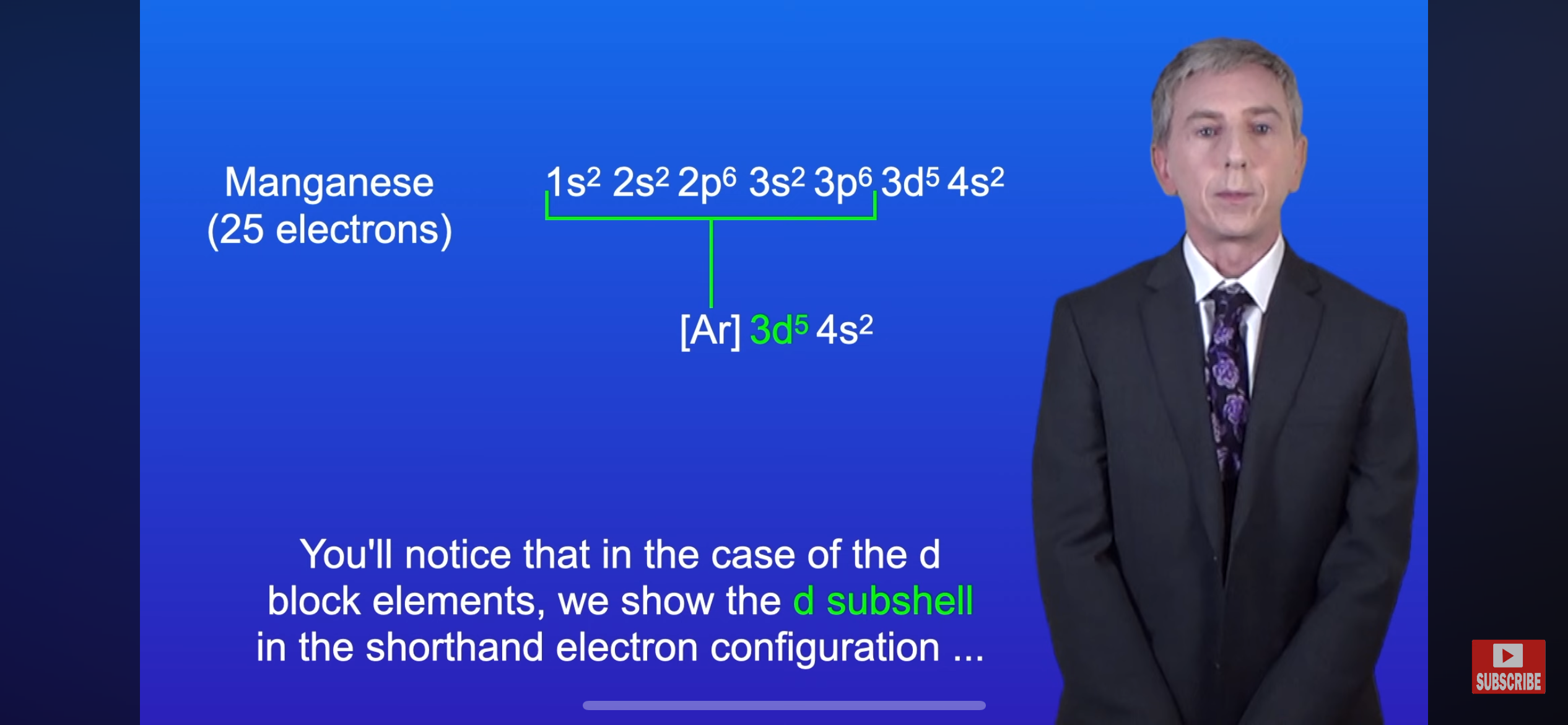
In the image, why is the d-sub-shell shown in the shorthand configuration?
Are we only allowed to use the noble gas that is in the period before the actual element of our electron configuration when doing the shorthand electron configuration?
Note:You use all the orbital types if it has the same number in the shorthand config because they are all part of the valence electrons(e.g. 3s2 and 3d4 would be on the shorthand config of sulfur since they are on the n=3 (outermost shell))
Because electrons in the d subshell can be involved in chemical reactions.
Yes.