Chemistry - Atomic Models, Orbital Diagrams, and Electron Configuration
1/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Democritus
first person to talk about atoms
created the Atomas Model
believed matter was “uncuttable”
John Dalton
1803 - proposed the Atomic Theory
created the Atomic Model
Atomic Theory
atoms are small particles that cannot be created, divided, or destroyed—-”indivisible”
atoms of the same element are identical
atoms of different elements have different characteristics
atoms join with other atoms to make new substances (compounds)
J.J Thomson
proved that an atom can be divided into smaller parts
experimented with cathode-ray tubes
discovered corpuscles (electrons)
proposed that atoms are electrically neutral
1897 - proposed the Plum Pudding Model
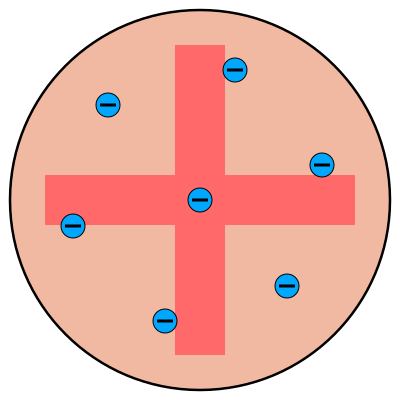
Plum Pudding Model
atoms mostly consist of positively charged material (“positive sea”)
negatively charged particles (electrons) are in fixed positions within the positive sphere
Cathode-Ray Experiment
led to the discovery of electrons
high voltage caused a beam of particles to shoot from the cathode
particles were attracted to the positive electric plate
particles deflected away from the negative side of the magnet
Ernest Rutherford
“Father of Nuclear Physics”
created the Nuclear Model of the atom
1909 - performed the Gold Foil experiment and suggested;
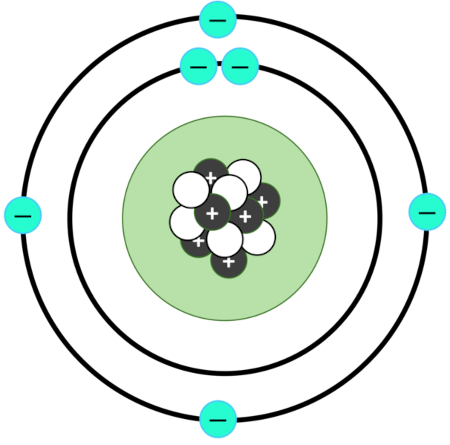
atoms consist of a small core (nucleus) that contains most of the mass of the atom
nucleus is made up of positively charged particles called protons
protons are surrounded by negatively charged electrons
atoms are mostly empty space
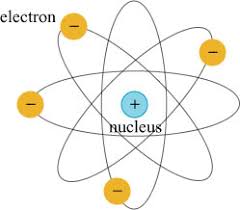
Nuclear Model
atom has a positively charged core
negatively charged electrons move around the nucleus
Gold Foil Experiment
disproved the Plum Pudding Model
radioactive source emitted a beam of positively charged alpha particles
the beam was directed at a thin sheet of gold foil
most alpha particles passed straight through; this proved that the atom is mostly empty space
small number of alpha particles repelled against the positive charge of the nucleus
Niels Bohr
1913 - developed the Planetary Model/Bohr Model
conducted light experiments
believed electrons can ‘jump’ from a path in one level to a path in another level depending on their energy
gaining energy = electrons move farther away from the nucleus
losing energy = electrons move closer to the nucleus
Bohr/Planetary Model
incorporated newer discoveries about how the energy of an atom changes when the atom absorbs or emits light
suggests that electrons travel around the nucleus of an atom in orbits or definite paths
orbits have specific sizes and energy levels
Light Experiments
when an electron absorbs light energy, it jumps to higher levels/orbits that are far from the nucleus
when an electron emits light energy, it falls to a lower level/orbit
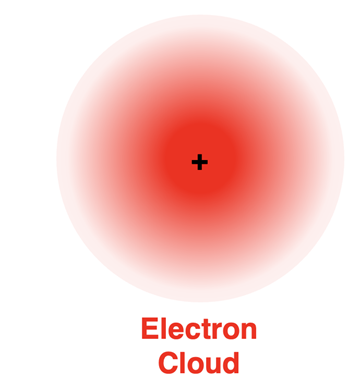
Erwin Schrodinger
created the Quantum Mechanical Model
1926 - further explained the nature of electrons by proposing that their location cannot be exactly stated
electrons move around the nucleus in regions called electron clouds/orbitals
electrons positioned closer to the nucleus are more likely to be found
Orbitals
orbit-like
can only hold two electrons
helps scientists predict the areas where electrons are located
Quantum Mechanical Model
the most accurate depiction of an atom
4 Sublevels
s sublevel
p sublevel
d sublevel
f sublevel
S Sublevel
contains 1 orbital
holds a maximum of 2 electrons
P Sublevel
contains 3 orbitals
holds a maximum of 6 electrons
D Sublevel
contains 5 orbitals
holds a maximum of 10 electrons
F Sublevel
contains 7 orbitals
holds a maximum of 14 electrons
Orbital Diagrams
displays how electrons are placed in atoms
each arrow represents an electron
Aufbau’s Principle
electrons occupy orbitals of lowest energy first
Pauli’s Exclusion Principle
an orbital can hold only 2 electrons
electrons must have opposite spin
Hund’s Rule
electrons fill all orbitals in a sub-level before doubling up
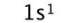
Electron Configuration
exponent represents the number of electrons
letter represents the sublevel