chem midterm
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Which of the following is not a chemical?
light
There is more traffic between 8 and 9 in the morning because most people start work at 9. This would be a(n)
observation
Write 540 000 in scientific notation.
5.4 × 105
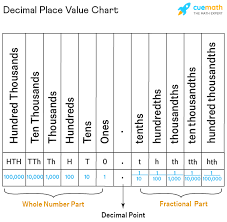
In the number 12.345, the 1 is in the_____ place
tens
When a part of the body is injured, substances called _____are released.
prostaglandins
The product of (-4) × (-5) is
+20
Write 0.000 000 33 in scientific notation.
3.3 × 10-8
Water, H20, is an example of a(n)
chemical
Which of the following is a chemical?
sugar
You notice that there is more traffic between 8 and 9 in the morning. This would be a(n)
observation
9.31 g is the same mass as
9310mg
A 50.0 mL urine sample has a mass of 50.7 g. The specific gravity of the urine is
1.014 g/mL
The measurement of the gravitational pull on an object is its
weight
1.00 pint of milk has a volume of how many milliliters?
473 mL
Which of the following measurements are not equivalent?
84 cm = 8.4 mm
In which of the following is the metric unit paired with its correct abbreviation?
milliliter / mL
The cubic centimeter (cm? or cc) has the same volume as a
milliliter
Which of the answers for the following conversions contains the correct number of significant figures?
24.95 min • 1 hr/ 60 min = 0.4158 hr
A piece of iron with a mass of 119 g is placed in a graduated cylinder, where the water level is to 57 mL. The water level rises to 72 mL. What is the density of the iron?
7.9 g/mL
A value of 345 mm is a measure of ____
length
If the heat of fusion for water is 80. cal/g, how many calories are needed to melt 45.0 g of ice at 0 degrees celsius
3.6 × 103 cal
The formation of a gas resulting from the escape of high-energy particles from the surface of a liquid is known as ____
evaporation
On a hot day, the thermometer read 95 °F. What is the temperature in degrees Celsius?
35 °C
One cup of kidney beans contains 15 g of protein, 1 g of fat, and 42 g of carbohydrate. How many kilocalories, to two significant figures, does this sample contain? (The caloric values are: 4 kcal/g for carbohydrate, 9 kcal/g for fat, and 4 kcal/g for protein. Round off the energy value for each food group to the tens place.)
240 kcal
A heating curve illustrates
the changes in the temperature and physical state of a substance as it is heated
Which of the following is a heterogeneous mixture?
reactions or mixtures where the reactants or components are not uniformly distributed, existing in different phases or having distinct boundaries
noodle soup
The heat of vaporization for water is 540 cal/g. How many kilocalories are needed to change 22g of liquid water to steam at 100 °C?
12 kcal
How many calories are required to increase the temperature of 13 g of ethanol from 11 °C to 23°C? The specific heat of ethanol is 0.59 cal/g °C.
92 cal
3.25 kcal is the same amount of energy as
A) 13600 J
The energy of motion is referred to as
kinetic energy
Metallic character increases going down a group.
true
Select the correct symbol for the element gold
Au
Which of the following gives the correct numbers of protons, neutrons, and electrons in a neutral atom of 118/50 Sn?
50 protons, 68 neutrons, 50 electrons
What is the mass number of an atom of potassium that has 20 neutrons?
39
Write the electron arrangement for the atom shown.
Phosphorus
1s22s22p63s23p3
The atomic number is equal to the number of protons.
true
The primary substances of which all other things are composed are
elements
A calcium atom two electrons in the fourth energy level.
true
Select the correct symbol for the element potassium.
K
What elements are in hydroxyapatite, Ca5(PO4)3OH, a major compound in human bones and teeth?
calcium, phosphorous, oxygen, helium
A sample of cerium-141 for a diagnostic test was dissolved in saline solution to an activity of 4.5 millicuries/mL. If the patient undergoing the test needs a dose of 10 millicuries, how much of the solution should be injected into the patient?
45 mL
One symptom of mild radiation sickness is
a lowered white cell count
The nuclear symbol that completes the equation is a(n)
13 13
N → C+?
7 6
positron
Krypton-79 has a half-life of 35 h. How many half-lives have passed after 105 h?
3 half-lives
The nuclear reaction shown below is an example of what type of process?
224 220 4
Th → Rn + He
90 88
alpha decay
The nuclear symbol that completes the equation is a(n)
59 59
Fe + ? —> Fe
26 26
neutron
Iron-59 has a half-life of 44 days. A radioactive sample has an activity of 0.64 mBq. What is the activity of the sample after 88 days?
0.16 mBq
Which of the following is suitable as a minimum shielding for beta particles?
1 cm of lead
85
For Sr, there are
38
38 protons and 47 neutrons
In the nuclear equation of beta decay,
the new nucleus contains 2 more protons
A group of covalently bonded atoms that has an overall electrical charge is called a(n)
polyatomic ion
In ionic compounds, ____lose their valence electrons to form positively charged
metals, cations
The main type of intermolecular forces between particles of NaCl are
ionic bonds
The shape of the methane molecule (CH4) is
tetrahedral
know these too:
linear
bent (109°) tetrahedral
trigonal planar
trigonal pyramidal
The shape of the water molecule (H20) is
bent (109°)
know these:
tetrahedral
trigonal pyramidal
linear
trigonal planar
Which one of the following compounds contains an ion with a 3+ charge?
FeCl3
The ammonia molecule (NH3) is
a polar molecule with nonpolar bonds
Which one of the following elements forms two or more ions with different ionic charges?
Fe
The main type of intermolecular forces between particles of carbon tetrafluoride (CF4) are
dispersion forces
The shape of a molecule with a central atom that has two bonded atoms and one lone pair is
bent (109°)
know:
trigonal planar
linear
bent (120°)
trigonal pyramidal
A reaction that releases energy as it occurs is classified as a(n)
exothermic reaction
How many moles of carbon atoms are there in 0.500 mole of C2H6?
1.00 mole
A chemical equation is balanced when
the number of atoms of each element is the same in reactants and products
consider the following equation. 2Mg + 02 →2MgO
How many grams of magnesium are needed to react with 16 g of O2?
24g
What is the classification for this reaction?
SO3 + H20 -H2504
combination
Pentane (C5H12) reacts with oxygen (O2) to form carbon dioxide (CO2) and water (H2O) according to the following reaction. Answer the following question(s) about this reaction.
C5H12 + ? 02 → CO2 + ? H20
What is the coefficient for carbon dioxide in the balanced equation?
5
How many moles of K2SO4 are in 15.0 g of K2SO4?
0.0861 moles
What type of reaction is: CH4+202 —>CO2 + 2H20 + 218 kcal?
an exothermic reaction
How many grams of K2SO4 are there in 0.123 mole of K2SO4?
21.4 g
For the following question(s), consider the following balanced equation.
Mg3N2 + 6H20 →3Mg(OH)2 + 2NH3
When 2 moles of Mg3N2 are allowed to react, how many moles of H2O also react?
12 moles
For the following question(s), consider the following balanced equation.
Mg3N2 + 6H20 →3Mg(OH)2 + 2NH3
Which of the following correctly describes the partial pressures of gases in the body?
high O2, low CO2, oxygenated blood
According to Avogadro's law,
the volume of a gas is directly related to the number of moles at constant temperature and pressure

A balloon is filled with helium gas. For the following question(a) elect the letter of the balloon diagram that corresponds to the given change in conditions.
Initial Volume: balloons
The temperature is changed from 50 °C to - 150 °C at constant pressure.
b
The pressure of 5.0 L of gas increases from 1.50 atm to 1240 mmg. What is the final volume of the gas, assuming constant temperature and amount of gas?
4.6 L
Which measurement describes the pressure of a gas?
725 mmHg
At constant temperature, a sample of helium at 760. torr in a closed container was compressed from 5.00 L to 3.00 L. What was the new pressure exerted by the helium on its container?
1270 torr
The pressure exerted by a gas on its container is directly proportional to
the number of molecules of gas in the sample
In response to Boyle's law, the pressure of a gas increases as the volume decreases because
C)the gas particles strike the walls of the container more often
A cyclopropane-oxygen mixture is used as an anesthetic. If the partial pressure of cyclopropane in the mixture is 330 mmg and the partial pressure of the oxygen is 1.0 atm, what is the total
1100 torr
In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules
occasionally come to rest
The compound KOH is
soluble, because all compounds containing OH are soluble
According to Henry's law, the solubility of a gas in a liquid
increases as the gas pressure above the liquid increases
What is the molarity of a solution which contains 58.5 g of sodium chloride dissolved in 0.500 L of solution?
2.00 M
An aqueous mixture containing starch (a colloid), NaCi, glucose, and albumin (a colloid) is placed 84) in a dialysis bag and immersed in distilled water. Which of the following correctly describes the location of the indicated substance after dialysis?
albumin inside
Rubbing alcohol is 70.% (m/v) isopropyl alcohol by volume. How many mL of isopropyl alcohol are in a 1 pint (473 mL.) container?
330 mL
What volume of 0.10 M NaOH can be prepared from 250. mL of 0.30 M NaOH?
750 L
A red blood cell will undergo crenation in
7% NaCl
What volume of a 1.5 M KOH solution is needed to provide 3.0 moles of KOH?
2.0 L
When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is
saturated
A homogeneous mixture that does not settle out upon standing is
a colloid

According to LeChâtelier's principle, predict whether adding H2O causes the system to shift in the direction of reactants, products, or no change.
(look at photo for equation)
products
The [H3O*] of a solution with pH = 8.7 is
2 × 10-9 M
The conjugate acid of H2O is
H30+
The neutralization reaction between Al(OH)3 and HNO3 produces the salt with the formula
Al(NO3)3
Which of the following is correctly identified?
H2CO2, strong acid
The [H30+] of a solution with pH = 2.0 is
1 × 10-2 M
If lung problems like emphysema occur, the blood pH of the patient is expected to
decrease
25.0 mL of 0.212 M NaOH is neutralized by 13.6 mL of an HCl solution. The molarity of the HCl solution is
0.390 M
The conjugate base of H20 is
OH-
A 10.0 mL of 0.121 M H2SO4 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
0.142 M