AQA Chemistry A-level, chemical equations to learn
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
full substitution (same size and charge)
[Cu(H2O)6]2+ + 6NH3 = [Cu(NH3)6]2+ + 6H2O
Partial substitution
[Cu(H2O)6]2+ + 4NH3 = [Cu(NH3)4(H2O)2]2+ + 4H2O
Het. catalyst - Haber process
N2 + 3H2 = 2NH3 (Fe catalyst)
Het. catalyst - Contact process
V2O5 + SO2 = V2O4 + SO3
V2O4 + 1/2O2 = V2O5(s)
Hom. catalyst - reacting peroxidisulfate and iodine
S2O8 2- + 2Fe 2+ = 2Fe 3+ + 2SO4 2-
2Fe 3+ + 2I- = I2 + 2Fe 2+
Autocatalysis - reacting oxalate (C2O4-) and MnO4-
MnO4- + 4Mn 2+ + 8H+ = 5Mn 3+ + 4H2O
2Mn 3+ + C2O4 2- = 2Mn 2+ + 2CO2
Cu2+ MAQI hydrolysis (water) to form weakly acidic solution and then further hydrolysis (OH- from water) to form insoluble metal hydroxides
[Cu(H2O)6]2+ + H2O = [Cu(OH)(H2O)5]+ + H3O+
[Cu(OH)(H2O)5]+ + H2O = [Cu(OH)2(H2O)4] (s) + H3O+
Amphoteric aluminium hydroxide + acid
Al(H2O)3(OH)3 + 3H3O+ = [Al(H2O)6]3+ + 3H2O
Amphoteric aluminium hydroxide + base
Al(H2O)3(OH)3 + OH- = [Al(H2O)2(OH4)]- + H2O
MAQI w little NH3
(NH3 + H2O = NH4+ + OH-) OH- formed so reacts the same as h2o to form insoluble metal hydroxides
Copper insoluble hydroxide + XS NH3
[Cu(OH)2(H2O)4] + 4NH3 = [Cu(NH3)4(H2O)2]2+ + 2OH- + 2H2O (deep blue solution)
Cu 2+ or Fe 2+ MAQI + CO3 2-
[M(H2O)6]2+ + CO3 2- = MCO3 (s) + 6H2O
AL 3+ or Fe3+ MAQI + CO32-
2[M(H2O)6]3+ + 3CO3 2- = 2[M(H2O)3(OH)3] (s) + 3CO2(g) + 3H2O
Exctract titanium from ore
TiCl4 + 2Mg = Ti + 2MgCl2
Al2O3 as acid
Al2O3 (s) + 2NaOH + 3H2O = 2NaAl(OH4) (1 2 3, 2)
Al2O3 as base
Al2O3 (s) + 3H2SO4 = Al2(So4)3 + 3H2O
SiO2 + 2NaOH
Na2SiO3 + H2O
P4O10 + 12NaOH
4Na3PO4 + H2O
SO2 (g) + 2NaOH
Na2SO3 + H2O
SO3(g) + 2NaOH
Na2SO4 + H2O
Ozone propagation
Cl. + O3 = ClO. + O2
ClO. + O3 = 2O2 + Cl.
Ozone overall
2O3 = 3O2
Cl2 + cold water
Cl2 + H2O = ClO- + Cl- + 2H+
Cl decomposing water in presencse of UV
2Cl2 + 2H2O = 4HCl + O2
Making bleach
Cl2 + 2NaOH = NaClO + NaCl + H2O
Group 7 reducing H2SO4
NaHSO4 SO2 S H2S
cl- br- --I- --
reducing nitrobenzene to phenylamine
C6H5NO2 (or draw) + 6[H] = C6H5NH2 + 2H2O
using LiAlH4 and dilute acid to reduce nitrile - how many mol reducing agent?
4[H]
Lithium electrode equation
Li+ + CoO2 + e- = Li+(coO2)-
hot alkali + chlorine
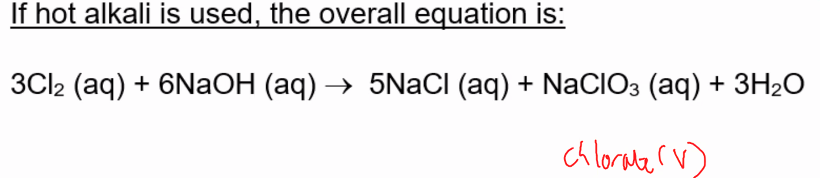
ionic equation for the reaction of iron(II) ions with manganate(VII) ions.
5Fe2+ + MnO4 − + 8H+ = Mn2+ + 5Fe3+ + 4H2O