PACKAGING MATERIALS QUALITY CONTROL
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Packaging Material
Defined as an economical means for providing presentation, protection, identification, convenience, and compliance for a product during storage, carriage, display, and until the product is consumed.
Primary Packaging Materials
Secondary Packaging Materials
Tertiary Packaging Materials
What are the Classes of Packaging Materials?
Ampoules
Vials
Plastic Bottles
Foils
Sachets
Example of Primary Packaging
Cartons
Labels
Leaflets
Example of Secondary Packaging Materials
Corrugated boxes
Shrink Film
Example of Tertiary Packaging Materials
Ideal Requirements of a Good Packaging
• They should be able to hold the product without loss on account of leakage, spoilage or permeation.
• They should protect against environmental conditions like light, air and moisture during storage.
• They should possess sufficient strength to withstand shocks of handling, transportation etc.
• They should facilitate efficient, safe and convenient use of contents.
• The material must not interact with the contents.
Glass
It is an amorphous solid material which is usually brittle and optically transparent.
Made of sand
Soda ash (Na2CO3)
Cullet
Silica (SiO2)
Alumina (Al2O3)
Boron
Potassium,
Zinc
Composition of Glass
Batching
Melting
Forming
Annealing
Processing methods of Glass
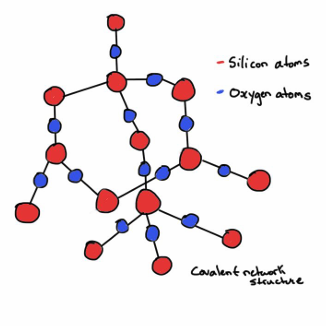
Silicon dioxide tetrahedron
Basic structural network of Glass
Migratory Oxides of Glass
Other oxides free to migrate which causes leaching and may hydrolyze to raise the pH of solution and catalyze or enter into reaction.
• Transparent/Clear
• Available in various shapes and sizes
• Can withstand the variation in temperature and pressure during sterilization
• Economical and easily available
• Protect photosensitive medicaments from light during storage.
• Can be easily labelled
• Can be sealed hermetically or by removable closures
Advantages of Glass
Prone to accidental breakage, Leaching, and Sorption
Disadvantage of Glass
Type I Glass: Highly Resistant, Borosilicate Glass
Type II Glass: Treated Soda Lime Glass
Type III Glass: Soda Lime Glass
Type IV or NP Glass: Non-Parenteral
Enumerate the type of Glass
Type I Glass: Highly Resistant, Borosilicate Glass
• Containing significant amounts of boric oxide, aluminum oxide, alkali and/or alkaline earth metal oxides (Silicon Dioxide, Sodium Oxide, Potassium Oxide, and Calcium Oxide)
• It has a high hydrolytic resistance and a high thermal shock resistance due to treatment of Sulfur Dioxide.

• Suitable for ALL products
• Usually intended for parenteral administration
Applications for Type I Glass: Highly Resistant, Borosilicate Glass
• High hydrolytic and thermal shock resistance
• Low leachability
• Low thermal coefficient of expansion
Characteristics of Type I Glass: Highly Resistant, Borosilicate Glass
Type II Glass: Treated Soda Lime Glass
• Usually made of soda lime glass with high hydrolytic resistance resulting from suitable treatment of the surface.
• Treated under controlled temperature and humidity conditions with sulfur dioxide.
• Usually for acidic or neutral solutions non-reactive with the glass.
• May be used for alkaline solution if stability data demonstrate its suitability.
Applications of Type II Glass: Treated Soda Lime Glass
Type III Glass: Soda Lime Glass
Silica glass containing alkali metal oxides; moderate hydrolytic resistance due to the chemical composition of the glass itself.
• Silicon dioxide
• Higher proportion of sodium oxide and calcium oxide compared to Type II and no boric oxide.
Chemical composition of Type III Glass: Soda Lime Glass
Type IV or NP Glass: Non-Parenteral
Soda lime glass NOT suitable as a container for parenterals
Type of Test: Powdered Glass
TEST FOR GLASS
Type I Glass: Highly Resistant, Borosilicate Glass
Use: Parenterals, Non-parenterals
Type III Glass: Soda Lime Glass
Use: Not for perenterals, unless tested suitable
Water attack
TEST FOR GLASS
Type II Glass: Treated Soda Lime Glass
Acidic and neutral aqueous products
Powdered Glass Test
• To evaluate the chemical resistance of glass formulations by measuring the amount of alkali released from glass powder.
• To differentiate Type I from Type III.
Water attack Test
•This test is used only with containers that have been exposed to sulphur dioxide fumes under controlled humidity conditions.
• Evaluation of the hydrolytic stability of the containers under more severe conditions.
• Amount of alkali released from the glass under the condition: 121 ± 2.0°C for 60 minutes; to determine whether the alkali leached from the surface of a container is within the specified limits or not.
• Polyethylene (low or high density)
• Polypropylene
• Polyvinyl chloride
• Polystyrene
• Polyethylene terephthalate
Plastic containers for pharmaceutical products are made from plastics based on the following polymers:
• Permeation
• Leaching
• Sorption
• Chemical Reactivity
Plastic Packaging Considerations