Hybridization of Atomic Orbitals
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Define hybridization.
the concept of mixing two or more atomic orbitals of an atom to form new, equivalent hybrid orbitals that are better suited for forming chemical bonds
If the electron pair geometry is linear (2e- groups), what is the hybridization?
sp
If the electron pair geometry is trigonal planar (3e- groups), what is the hybridization?
sp^2
If the electron pair geometry is tetrahedral (4e- groups), what is the hybridization?
sp^3
If the electron pair geometry is trigonal bipyramid (5e- groups), what is the hybridization?
sp^3d
If the electron pair geometry is octahedral (6e- groups), what is the hybridization?
sp^3d^2
How many unhybridized p-orbitals are there in sp^3?
None
How many unhybridized p-orbitals are there in sp^2?
1
How many unbridized p-orbitals are there in sp?
2
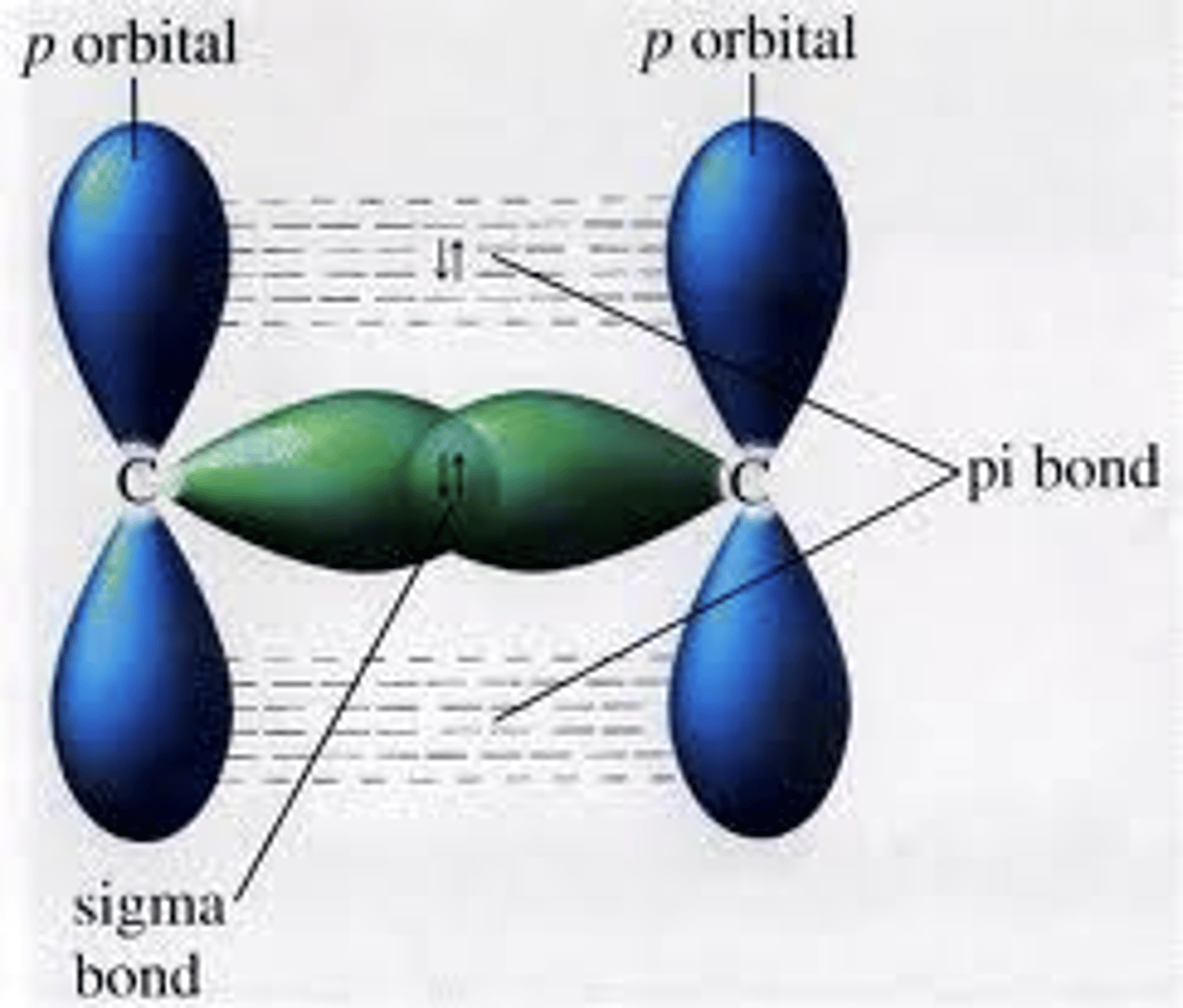
Define sigma bonds.
A bond is formed when the ends of orbitals overlap (directly between nuclei)
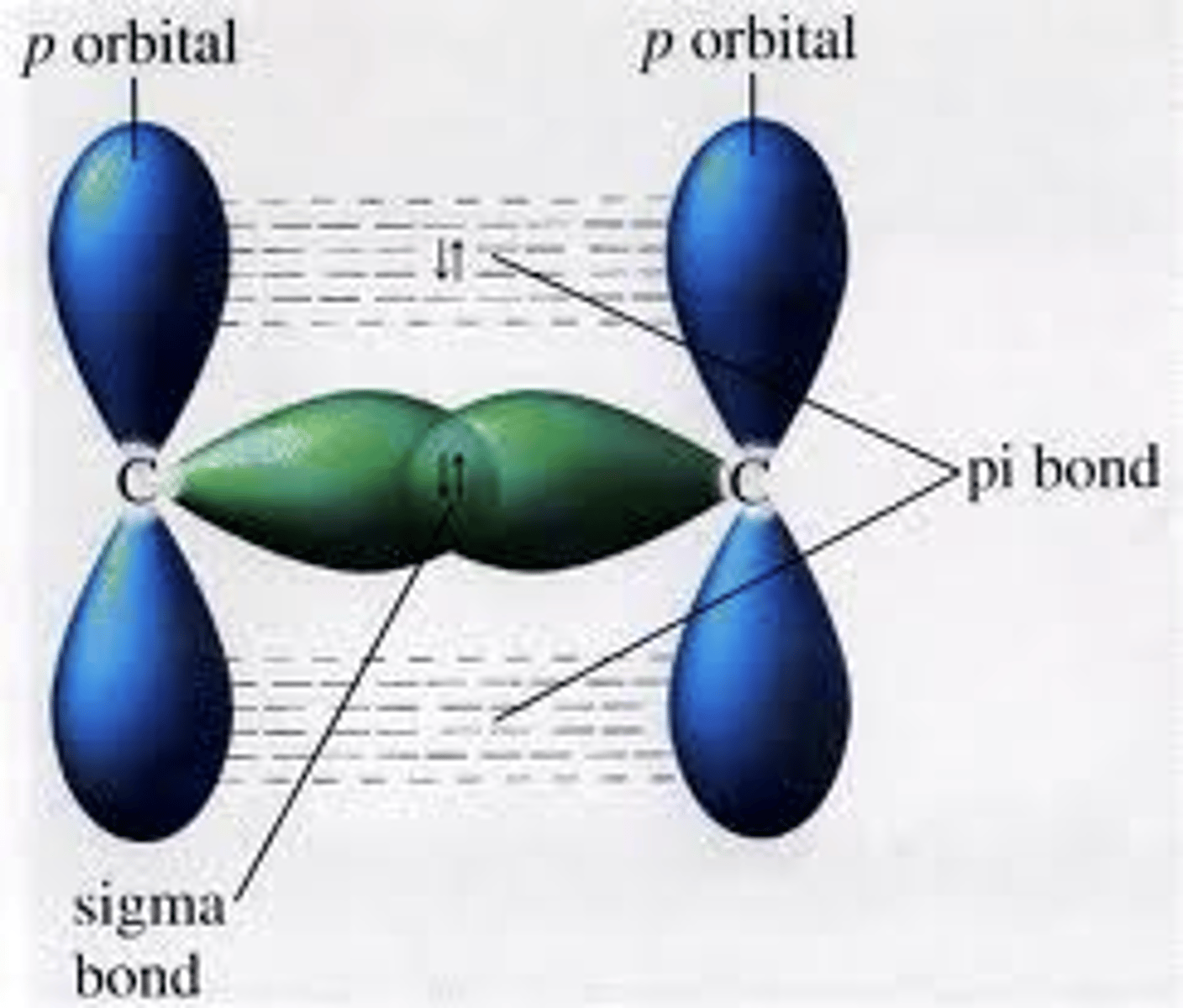
Define pi bonds.
A bond formed when the sides of orbitals overlap (above and below line between nuclei)
What are the two roles of a hybrid orbital?
1. Make sigma bonds
2. Hold lone pairs
Do all bonds contain 1 sigma bond?
Yes
How are pi bonds made?
Unhybridized p orbitals
For nitrogen is have a formal charge of 0, how many bonds and lone pairs does nitrogen need to have?
3 bonds and 1 lone pair
For carbon to have a formal charge of 0, how many bonds and lone pairs does carbon need to have?
4 bonds and 0 lone pairs
For oxygen to have a formal charge of 0, how many bonds and lone pairs does oxygen need to have?
2 bonds and 2 lone pairs