Gibs Free energy
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
what does gibs free energy determine
determines reactions feasibility
How to calculate entropy change of surroundings
-ΔH / T
units of ΔS surroudnings
KJ K-1 mol-1 x 100 = JK-1mol-1
how to calculate gibs free energy
ΔG = ΔH -TΔS
what does negative ΔG mean
feasable
units for equation for ΔG
all Δ - KJ
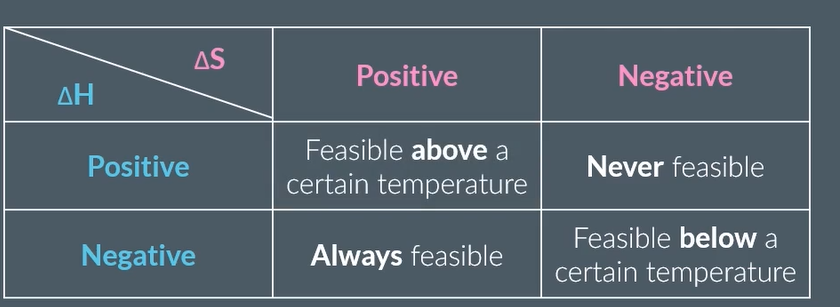
different feasibility to negatives and positives ΔG and Δ S
how to work out ΔS using graph
gradient → reverse graph → convert JK-1mol-1
how to work out ΔH using graph
using y intercept
how to determine temperature of feasibility using graph
when line is below 0
how to determine temperature of feasibility using equation
set ΔG equal to 0
ensure ΔH and ΔS are in KJ-1mol-1
why would a reaction be feasible but nothing happen
Activation energy too low so rate of reaction too slow