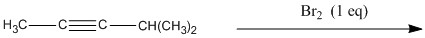Ch 8, 9, 10 Pearson
1/155
Earn XP
Description and Tags
Organic Chemistry summer
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
156 Terms
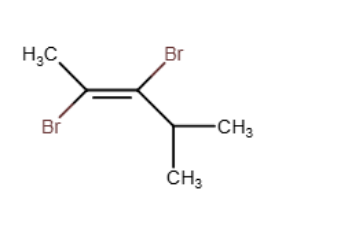
Provide the structure of the major organic product of the reaction below.
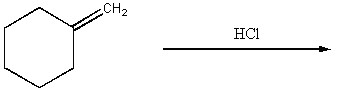
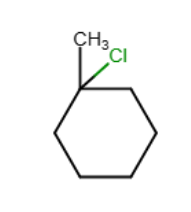
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
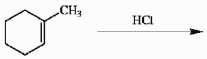
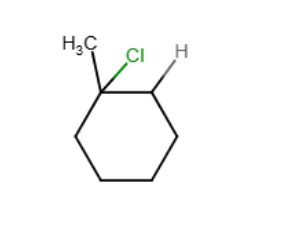
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
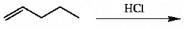
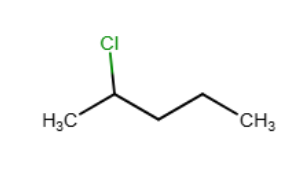
HBr can be added to an alkene in the presence of peroxides (ROOR). What function does the peroxide serve in this reaction?
radical chain initiator
Name the major product which results when HBr is added to 3-ethyl-3-hexene.
3-bromo-3-ethylhexane
Provide the structure of the major organic product of the reaction below.
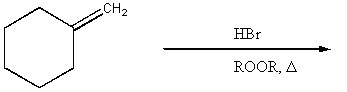
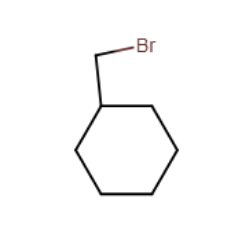
Provide the major organic product of the reaction below.
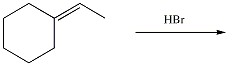
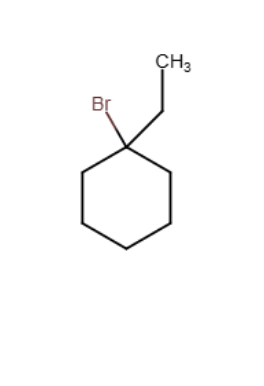
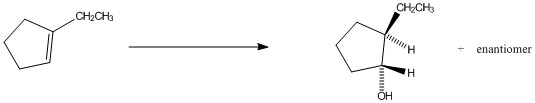
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
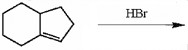
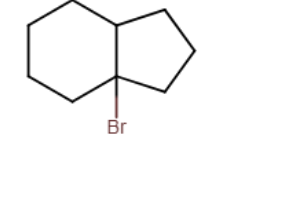
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
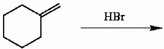
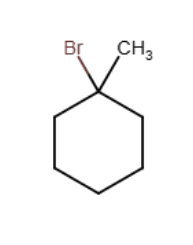
When propylene reacts with hydrogen bromide in the presence of a peroxide initiator, which of the following structures are formed during the mechanism?

Draw the major regioisomeric product generated in the reaction below.
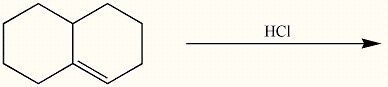
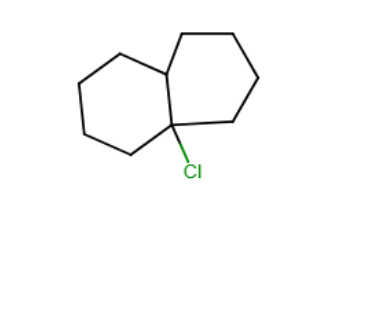
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
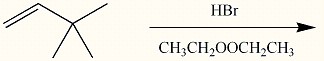
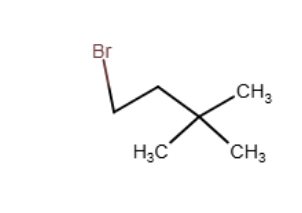
Acid catalyzed hydration (H2SO4/water/△) of an unknown compound (A)with a chemical formula C6H12, yielded a racemic mixture of product C6H13OH. Which, if any, of the following compounds is/are possible structures for the initial compound (A)?
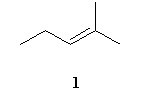 | 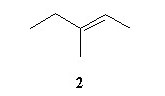 | 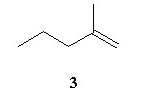 |
none of the above
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
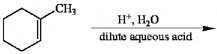
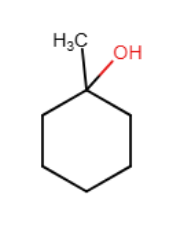
What is the major product of the following reaction?

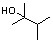
Which of the following intermediates is thought to occur in the mechanism by which alkenes are hydrated in the presence of acid?
carbocation
Complete the following reaction.
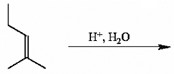
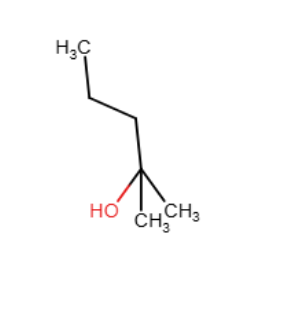
Which of the following is the best reaction sequence to use if one wants to accomplish a Markovnikov addition of water to an alkene with minimal skeletal rearrangement?
oxymercuration-demercuration
What synthetic goal is achieved by subjecting an alkene to an oxymercuration-demercuration sequence?
Markovnikov addition of H2O wherein skeletal rearrangement is prevented
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
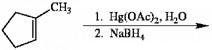
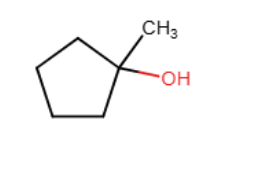
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
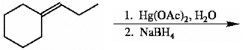
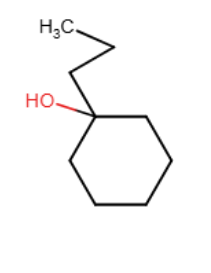
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
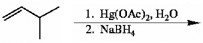
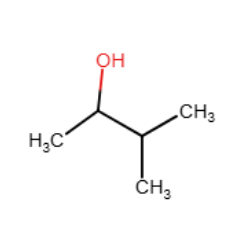
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
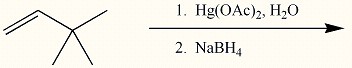
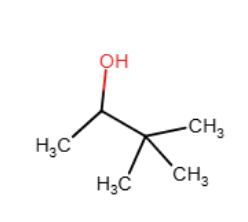
When an alkene is subjected to treatment with Hg(OAc)2 in alcohol followed by reaction with NaBH4, what new class of compound is formed?
ether
Treatment of 2-methylpropene with which of the following reaction conditions results in an anti-Markovnikov addition product?
both "dry gaseous HBr with peroxides present" and "BH3-THF, followed by alkaline H2O2" |
Provide the structure of the major organic product of the reaction below.
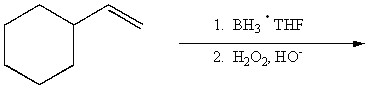
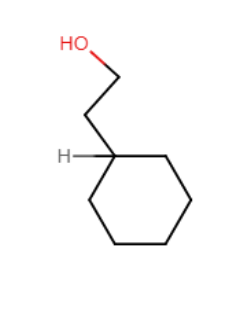
Provide the structure of the major organic product of the reaction below.
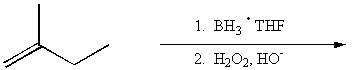
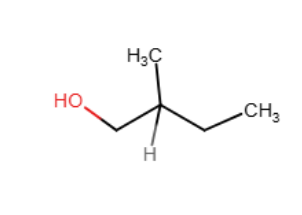
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
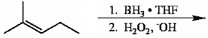
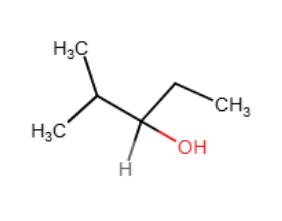
Provide the reagents necessary to complete the following transformation.
1)BH3∗THF2)H2O2,HO−1)BH3∗THF2)H2O2,HO−
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
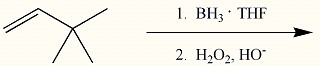
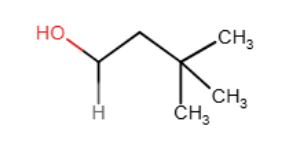
Provide the major organic product of the reaction below.
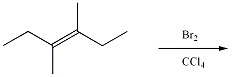
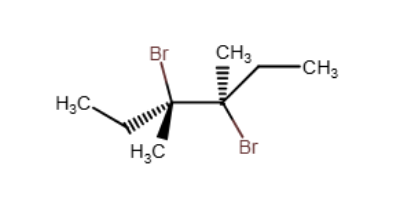
Which of the following alkenes will yield a meso dihalide when reacted with Br2/CCl4 at room temperature?
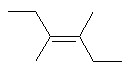
Addition of Br2 to (EE)-hex-3-ene produces ________.
a meso dibromide
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
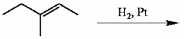
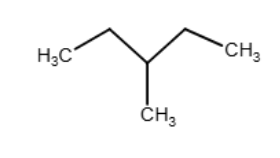
Both (EE)- and (ZZ)-hex-3-ene can be treated with D2 in the presence of a platinum catalyst. How are the products from these two reactions related to each other?
The products of the two isomers are related as diastereomers.
________ is the use of an optically active reagent or catalyst to convert an optically inactive starting material into an optically active product.
Asymmetric induction
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
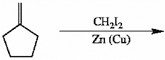
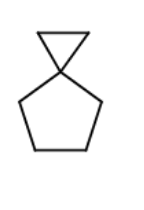
Complete the following reaction by filling in the necessary reagents.
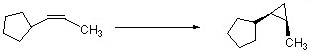
CH2I2/Zn(Cu)
Provide the reagents necessary to complete the following transformation.
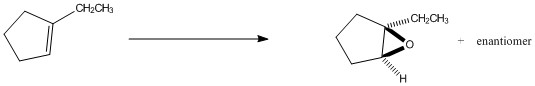
mCPBA
Treatment of cyclopentene with peroxybenzoic acid ________.
yields a meso epoxide
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
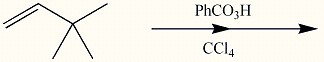
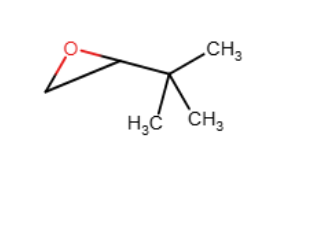
Provide the reagents necessary to complete the following transformation.
1)CH3CO3H2)H+,H2O
Provide the reagents necessary to complete the following transformation.
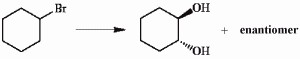
1) NaOH 2) H₂O, H⁺
Provide the reagents necessary to complete the following transformation.

OsO4,H2O2
Which of the following additions to alkenes occur(s) specifically in an syn fashion?
dihydroxylation using OsO4, H2O2, addition of H2, and hydroboration
Provide the major organic product of the following reaction.
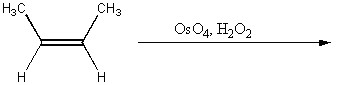
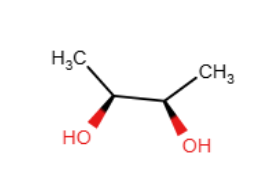
Provide the reagents necessary to complete the following transformation.
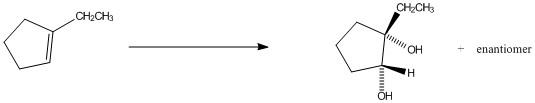
OsO4,H2O2
An unknown compound with empirical formula C3H5 was treated with Br2/CCl4. The bromine solution went from orangish/red to clear immediately at room temperature. Upon treatment with O3 followed by work-up with dimethylsulfide the following products were identified. From the information provided what is/are the most likely structure(s) for this unknown compound.
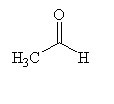 | 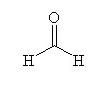 | 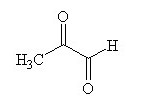 |
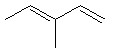
How many moles of carbon dioxide are generated when one mole of the compound shown is treated with an excess of warm, concentrated KMnO4 solution?
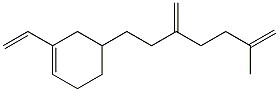
3
Provide the major organic product of the reaction below.
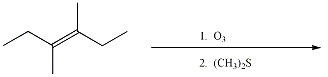
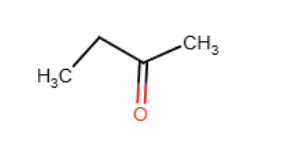
Draw the major organic products (two) generated in the reaction below. Pay particular attention to regio- and stereochemical detail.
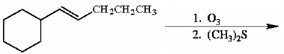
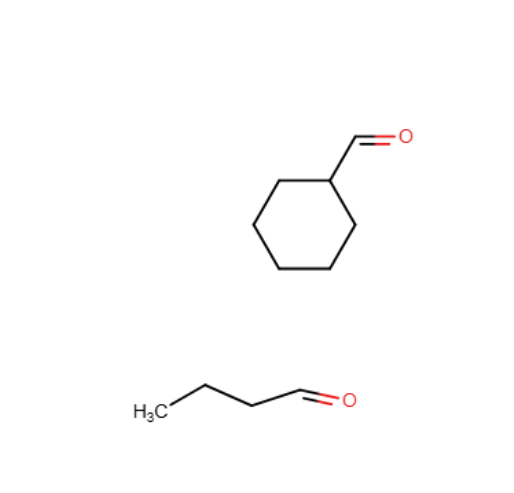
Which of the following compounds is (are) appropriate to promote the cationic polymerization of isobutylene?
both H2SO4 and BF3
How many distinct alkynes exist with a molecular formula of C4H6?
2
How many distinct alkynes exist with a molecular formula of C4H8?
0
Give the new IUPAC name for HC≡≡CCH2CH2CH3.
pent-1-yne
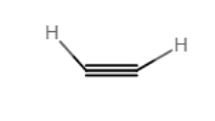
Draw an acceptable structure for acetylene.
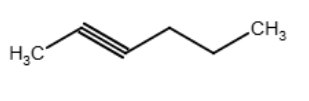
Draw an acceptable structure for hex- 2-yne.
How many distinct terminal alkynes exist with a molecular formula of C5H8?
2
How many distinct internal alkynes exist with a molecular formula of C6H10?
3
Give the new IUPAC name for Cl3CCH2CH2CH2C≡CH.
6,6,6-trichlorohex-1-yne
Provide the IUPAC name for the compound below.
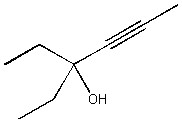
3-ethylhex-4-yn-3-ol
Provide the IUPAC name for Cl3C(CH2)3C≡CH.
6,6,6-trichlorohex-1-yne
Name the compound shown below.

6,6-dimethylhept-3-yne
Give the IUPAC name for CH3CH2C≡CCH(OH)CH3.
hex-3-yn-2-ol
Draw an acceptable structure for 3-sec-butylhept-1-yne.
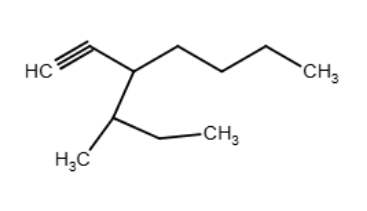
Draw an acceptable structure for hepta-3,6-dien-1-yne.
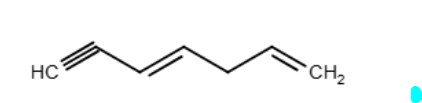
What is the correct IUPAC name for the following compound?
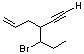
3-(1-bromopropyl)-5-hexen-1-yne
Give the IUPAC name for CH3CH=CHCH=CHC≡CCH3.
octa-2,4-dien-6-yne
Which of the following improperly describes the physical properties of an alkyne?
insoluble in most organic solvents
Which of the following alkynes has the lowest boiling point?
3,3-dimethyl-1-butyne
How many moles of oxygen are required in the complete combustion of 1 mole of acetylene?
2.5
The pi bond of an alkyne is ________ and ________ than the pi bond of an alkene.
shorter, weaker
The carbon-carbon triple bond of an alkyne is composed of ________.
one σ bond and two π bonds
In trans-hept-4-en-2-yne the shortest carbon-carbon bond is between carbons ________.
2 and 3
Which of the species below is less basic than acetylide?
CH₃ONa
Among the compounds water, but-1-yne, but-2-yne, and ethane, which are stronger acids than ammonia?
water and but-1-yne
To a solution of propyne in diethyl ether, one molar equivalent of CH3Li was added and the resulting mixture was stirred for 0.5 hour. After this time, an excess of D2O was added. Describe the major organic product(s) of this reaction.
CH3C≡CD + CH4
Which of the following bases are sufficiently strong to deprotonate a terminal alkyne with an equilibrium constant greater than 1?
1) sodium methoxide 3) nn-butyl lithium
2) lithium diisopropyl amide 4) phenylmagnesium bromide
2, 3 and 4
What conditions could be used to isomerize hept-2-yne to hept-1-yne?
1.NaNH2, 150 °C
2.H2O
Why is a terminal alkyne favored when sodium amide (NaNH2) is used in an elimination reaction with 2,3-dichlorohexane?
The strong base deprotonates the terminal alkyne and removes it from the equilibrium
When 2,2-dibromobutane is heated at 200°C in the presence of molten KOH, what is the major organic product?
but-2-yne
Describe a sequence of reactions by which hept-1-yne can be straightforwardly prepared from hept-1-ene.
1) Br2; 2) NaNH2, heat.
Complete the short synthesis below by providing the necessary reagents.

1)Br2;2)CH3C≡CNa;3)Na/NH3
Which of the following describes an unsymmetrical addition reaction?
propyne with 1 mole HBr |
Which of the following reagents will convert 1 mole of 3-methylpent-1-yne into 3-methylpentane?
2 moles H2, Ni and heat
Describe a sequence of reactions by which trans-pent-2-ene can be straightforwardly prepared from propyne.
1) NaNH2; 2) CH3CH2Br; 3) Na, NH3.
Which of the following reagents should be used to convert hex-3-yne to (EE)-hex-3-ene?
Na, NH3
Which of the following reagents should be used to convert hex-3-yne to (ZZ)-hex-3-ene?
H2, Lindlar's catalyst
A mixture of hept-1-yne, hept-2-yne, and hept-3-yne was hydrogenated in the presence of a platinum catalyst until hydrogen uptake ceased. If one assumes that the hydrogenation went to completion, how many different seven-carbon hydrocarbons were produced?
1
________ is produced when 1 equivalent of HBr is added to hex-1-yne in the presence of peroxides.
A mixture of E and Z isomers of 1-bromohex-1-ene
Which of the alkyne addition reactions below involve(s) an enol intermediate?
both hydroboration/oxidation and treatment with HgSO4 in dilute H2SO4
What is the major organic product that results when 1-heptyne is treated with 2 equivalents of HBr?
2,2-dibromoheptane
What class of organic product results when 1-heptyne is treated with a mixture of mercuric acetate in aqueous sulfuric acid?
ketone
What class of organic product results when 1-heptyne is reacted with disiamylborane followed by treatment with basic hydrogen peroxide?
aldehyde
Name the compound which results when pent-1-yne is treated with sodium in liquid ammonia.
pent-1-ene
Provide the major organic product of the reaction shown below.
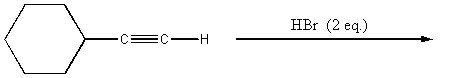
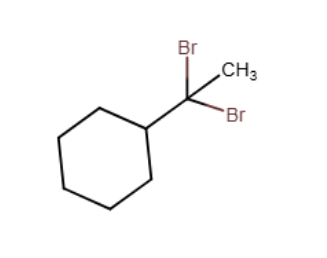
Provide the major organic product of the reaction shown below.
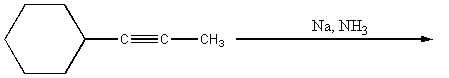
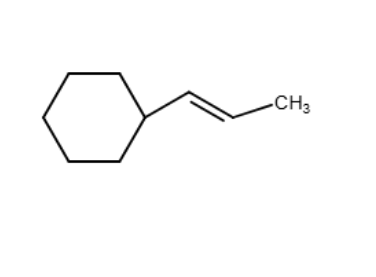
Provide the structure of the major organic product(s) in the reaction below.
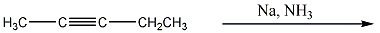
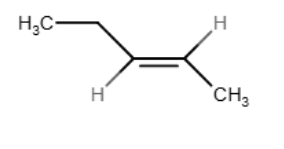
Provide the structure of the major organic product(s) in the reaction below.
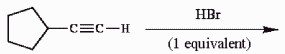
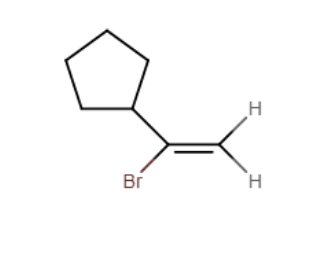
Provide the major organic product of the reaction shown below.