Thermodynamics, Kinetics, and Gas Laws
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Hund’s rule
“Empty Seats First!”
Think of electrons on a bus—they don't like sitting together unless they have to!
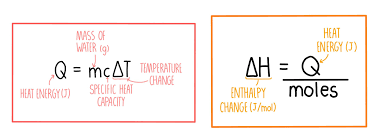
Molar Enthalpy (ΔH)
“One Mole, One Goal—Track the Heat Toll!”
"Molar enthalpy counts the heat when a mole makes its move—whether melting, boiling, or bonding."
Energy in Bond Breaking and Forming
"Chemical changes trade energy—breaking costs, forming pays back."
“Break Takes, Form Frees!”
Exothermic Reactions — Bond Energies
If bond breaking energy is less than bond forming energy than the reaction is exothermic
Surrounding temperature rises due to excess energy being released as heat
Endothermic Reactions — Bond Energies
Endothermic reactions are energy thirsty—they sip heat from the surroundings to stay balanced.
heat energy is pulled in → surroundings cool down
Enthalpy Change (ΔH)
Represents the heat energy absorbed or released by a reaction at constant pressure
Positive vs. Negative ΔH
Positive ΔH → Endothermic (absorbs heat from surroundings)
Negative ΔH → Exothermic (releases heat to surroundings)
Change in Total Energy and Enthalpy
"Enthalpy Equals Energy—Especially in Easy Pressure."
Highlights that under easy (constant) pressure, enthalpy change mirrors energy change.
Hess’ Law
"Hess doesn't stress—he just adds the steps!"
With Hess’ Law, you just sum up the enthalpy changes of the individual steps to get the total.
Manipulating Reactions for Hess’ Law
"Flip the sign, scale the line." → If you reverse a reaction, flip the sign of ΔH. If you scale it, stretch ΔH the same way.
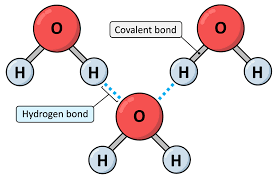
Hydrogen Bonding
Hydrogen bonding is a strong intermolecular force that occurs when a hydrogen atom, covalently bonded to a highly electronegative atom like N, O, or F, is attracted to another electronegative atom nearby.
🌊 It’s responsible for many unusual properties of water, like high boiling point and surface tension.
Why do structures with greater intermolecular forces have higher boiling points?
Because stronger intermolecular forces require more energy to overcome, meaning more heat is needed for molecules to separate and enter the gas phase—resulting in a higher boiling point.
Types of Forces:
Dispersion (London)
Dipole-dipole
Hydrogen bonding
Ion-dipole
🌡 Boiling point increases with strength of intermolecular attraction.
What type of enzyme is a kinase?
Kinases are transferases that move phosphate groups
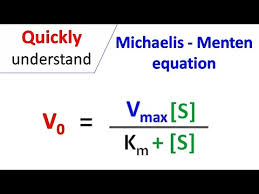
What is the equation for Michelis Menten and what does it represent? (Michaelis Mentens the speed)
it's about reaction velocity depending on substrate availability.
v = Vmax [S] / Km + [S]
What does the kcat/Km ratio represent in enzyme kinetics? (“Efficiency = Speed ÷ Affinity Cost”)
Reflects how efficiently an enzyme converts substrate to product at low substrate concentrations
What does kcat measure in enzyme kinetics?
called the turnover number
kcat is the rate constant for the catalytic step of an enzyme reaction
A higher kcat means the enzyme is more efficient at converting substrate to product
What does Km indicate in enzyme kinetics?
Km is the substrate concentration at which the enzyme operates at half its maximum speed
It reflects the affinity of the enzyme for its substrate
What does a high and low Km mean?
Low Km → high substrate affinity (enzyme binds substrate tightly)
High Km → low substrate affinity (enzyme requires more substrate to be effective)
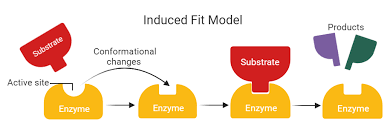
What is the induced-fit model in enzyme catalysis?
The induced-fit model proposes that an enzyme’s active site is flexible, not a rigid lock
Upon substrate binding, the enzyme undergoes a conformational change that aligns catalytic groups for reaction
What is catalysis?
A process where a catalyst speeds up a chemical reaction by lowering activation energy
What does traditional Michaelis–Menten kinetics describe about initial velocity (V₀) and substrate concentration ([S])?
More Substrate, More Speed—Then a Plateau Indeed”
The initial velocity (V₀) of an enzyme-catalyzed reaction shows a hyperbolic dependence on substrate concentration ([S])
As [S] increases, V₀ rises rapidly at first, then levels off as the enzyme becomes saturated
How does an uncompetitive inhibitor affect Km and Vmax in enzyme kinetics?
“U-turn for Both: Km & Vmax Down”
What is an uncompetitive inhibitor and how does it affect enzyme kinetics?
An uncompetitive inhibitor binds only to the enzyme-substrate complex (ES)
It forms an inactive enzyme-substrate-inhibitor (ESI) complex
This prevents the enzyme from converting substrate into product
What are the key components of a Lineweaver–Burk plot in enzyme kinetics?
Intercepts:
Y-intercept: 1/Vmax
X-intercept: −1/Km