1. elements of life
5.0(1)
Card Sorting
1/138
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
139 Terms
1
New cards
electrons
negatively charged particles which move around the nucleus in shells
2
New cards
nucleus
central part of the atom with most the mass made up of protons and neutrons
3
New cards
mass number
larger number which is the total of protons and neutrons
4
New cards
atomic number
smaller number which is the number of protons or electrons
5
New cards
negative ions
have more electrons than protons
6
New cards
postive ions
have fewer electrons than protons
7
New cards
isotope
elements with the same atomic number but different mass number due to different amounts a neutrons, with the same chemical properties but different physical properties (density, diffusion)
8
New cards
ancient greeks
though that all mater was made from indivisible particles
9
New cards
John dalton
at the start of the 19th century it was suggested atoms were solid spheres
10
New cards
JJ thomson
in 1897 experiments concluded that atoms must have smaller negatively charged particles, electrons, he created the plum pudding model
11
New cards
Ernest Rutherford
Geiger-Marsden experiment where alpha particles were fired at gold sheet and where deflected and some went through, nuclear model with a positive centre and cloud of electrons
12
New cards
Henry Moseley
charge of nucleus increased by one form element to element, lead to Rutherford discovering the proton
13
New cards
James Chadwick
conclude there are neutrons with a mass but no charge
14
New cards
Bohr model (Niels Bohr)
proposed that elections are in fixed orbits with a fixed energy, when electrons move shells they emit or absorb electromagnetic radiation
15
New cards
quantum model
model which uses quantum mechanics to predict where electrons will be
16
New cards
relative mass
the molecular mass is relative to carbon 12
17
New cards
mass spectrometer
1. sample is vaporised
2. ionisation to positive ions by bombardment of high energy electrons
3. the ions are accelerated by an electric shield
4. time for the ions to reach detector is measured with lighter ones been quicker
18
New cards
calculate relative atomic mass
(abundance x mass) ÷ 100
19
New cards
mole
amount of a substance
20
New cards
avogadros constant
6\.02 x10 ^23
21
New cards
number of moles
= number of particles you have ÷ avogadros constant
22
New cards
number of moles
= mass ÷ Mr
23
New cards
concentration
= moles ÷ volume
24
New cards
empirical formula
smallest whole number ratio of a compound, worked out from moles and then dividing by smallest
25
New cards
water of crystallisation
compounds with incorporated water
26
New cards
hydrated
compounds containing water of crystallization
27
New cards
anhydrous
compounds without incorporated water
28
New cards
calculate water of crystallization
find moles of water lost from mass
find moles of compound from mass
divide by smallest
find moles of compound from mass
divide by smallest
29
New cards
percentage yield
actual yield ÷ theoretical yield x 100
30
New cards
standard solution
a solution with a known concentration
31
New cards
6, 3, 4, 9, 8, 2, 7, 1, 5
order statements making standard solution
1. place stopper in bottle and invert
2. rinse the beaker and stirring rod with distilled water and add it to flask
3. work out how many grams of solute needed using mass = moles x mr
4. measure out the mass of the solute first measuring beaker
5. check meniscus again and add water if needed
6. work out moles of solute using using moles = conc x volume ÷ 1000
7. top up flask with distilled water till at meniscus using pipette
8. top up solution into volumetric flask using funnel
9. add distilled water to beaker to dissolve solute
1. place stopper in bottle and invert
2. rinse the beaker and stirring rod with distilled water and add it to flask
3. work out how many grams of solute needed using mass = moles x mr
4. measure out the mass of the solute first measuring beaker
5. check meniscus again and add water if needed
6. work out moles of solute using using moles = conc x volume ÷ 1000
7. top up flask with distilled water till at meniscus using pipette
8. top up solution into volumetric flask using funnel
9. add distilled water to beaker to dissolve solute
32
New cards
diluting solution
making a standard solution from more conc solution
33
New cards
volume to use
final conc ÷ initial conc x volume required
34
New cards
1, 4, 7, 2, 3, 5, 6,
1. add acid to burette to the 0cm3 line
2. carry out rough titration to find the endpoint
3. work out amount of acid used
4. add alkali to flask using 25cm3 pipette
5. carry out accurate titration till you get concordant results
6. calculate mean
7. add indicator to flask
35
New cards
methyl orange
turns yellow to red/peach when adding acid to alkali
36
New cards
phenolphthalein
turns red to colourless when adding acid to alkali
37
New cards
s
sub shell with 2 electrons
38
New cards
p
sub shell with 8 electrons
39
New cards
d
sub shell with 10 electrons
40
New cards
f
sub shell with 14 electrons
41
New cards
spin pairing
electrons spin in opposite directions
42
New cards
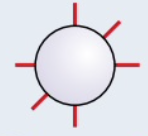
s orbital
spherical orbitals
43
New cards
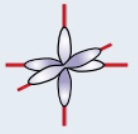
p orbital
dumbbell shaped orbital, made up of 3
44
New cards
s block
group 1 and 2
45
New cards
d block
transition metals
46
New cards
p block
group 3-8
47
New cards
ionic bonding
electrons are transferred making 2 ions and then hold each other together by electrostatic attractions, non metal and metal
48
New cards
dot and cross
diagram showing electron distribution in shells, use square brackets and charge for ionic compounds, shells overlap for covalent
49
New cards
giant ionic lattice
compounds which form crystals with regular repeating structure
50
New cards
ionic properties
conduct when molten or dissolved
high melting points
soluble in water
high melting points
soluble in water
51
New cards
covalent bonds
share electrons been 2 non metals, help by electrostatic attraction between nuclei and electrons as well as repulsion between nuclei
52
New cards
dative covalent bonding
covalent bond where both bonding electrons are from one element
53
New cards
giant covalent structures
structures like carbon and silicon dioxide
54
New cards
properties of giant covalent structures
very high melting point
very hard
good thermal conductors
don’t dissolve
can’t conduct
very hard
good thermal conductors
don’t dissolve
can’t conduct
55
New cards
metallic bonding
bonding between metals where they lose electrons making them delocalised leaving a positive ion, attraction between ion and electrons hold them together
56
New cards
metallic bonding properties
high melting points due to sea of delocalised electrons
ions able to slide over each other
good thermal and electrical conductors due to sea of delocalised electrons
insoluble due to high bond strength
ions able to slide over each other
good thermal and electrical conductors due to sea of delocalised electrons
insoluble due to high bond strength
57
New cards
shape
depends on the number pf pairs of electrons in outer shell
58
New cards
electron effect
pairs of electrons are negative and will repel each other to get as fair apart from each other as possible
59
New cards
lone pairs
pairs of electrons that repel more than bonding pairs
60
New cards
wedges
shows bonds pointing out page
61
New cards
dashes
shows bonds pointed into page
62
New cards
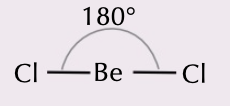
linear molecules
2 bonding pairs bond angle of 180 degrees
63
New cards
trigonal planar
3 bonding pairs, bond angle of 120˚
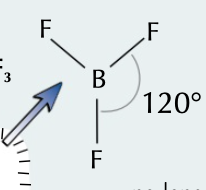
64
New cards
non linear
2 bonding pairs with double bonds cancel out effect of 1 lone pair, bond angle of 120˚
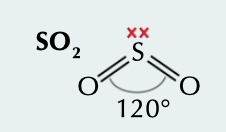
65
New cards
tetrahedral
4 bonding pairs, bond angle of 109.5˚
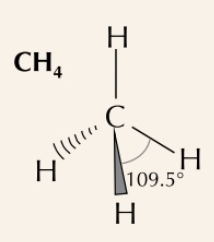
66
New cards
trigonal pyramidal
3 bonding pairs, 1 lone pair, bond angle of 106˚
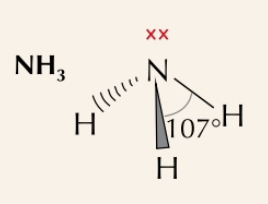
67
New cards
bent
2 bonding pairs, 2 lone pairs, bonding angle of 104.5˚
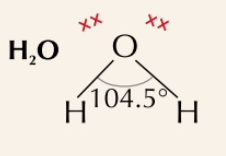
68
New cards
trigonal bipyramidal
5 bonding pairs, bonding angle of 120˚ and 90˚
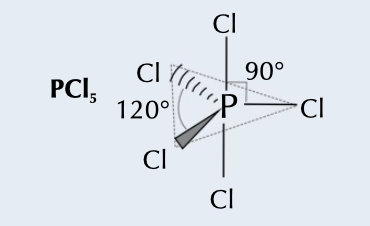
69
New cards
octahedral
6 bonding pairs, bonding angle of 90˚d
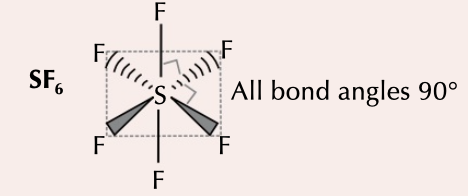
70
New cards
determine shapes
draw a dot and cross diagram, allows you to see numbers of bonding and lone pairs
71
New cards
calculate shape
1. find group number of central atom (only one of)
2. add number of atoms around atom to its group number
3. divide by 2 to get electron pairs
if there is the same number of electron pairs as surrounding atoms then no lone pairs.
if ion then subtract or add its charge from its group
72
New cards
group
column where all elements have the same number of electrons in outer shell
73
New cards
period
row where all elements have the same number of shells
74
New cards
melting points
a trend which increases across a period peaking at group 4, related to number of delocalised electrons, giant covalent structures and intermolecular forces
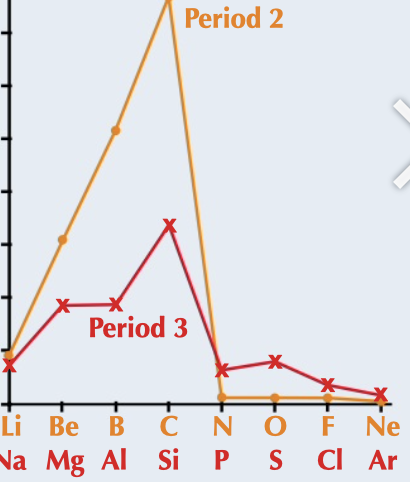
75
New cards
first ionisation enthalpy
energy needed to remove 1 electron from each atom in one mole of gaseous atoms to become 1 mole of gaseous 1+ ions
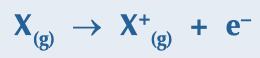
76
New cards
lower enthalpy
easier it is to remove on outer electron and form an ion
77
New cards
factors effecting first ionisation enthalpies
atomic radius- dist electrons from nucleus
nuclear charge- more positive nucleus attracts electrons more
electron shielding- inner electrons shield outer electrons.
nuclear charge- more positive nucleus attracts electrons more
electron shielding- inner electrons shield outer electrons.
78
New cards
group 1 and 2 first ionisation enthalpy
trend which decreases down a group due to increasing shells causing shielding
79
New cards
across a period
direction where first ionisation enthalpy increases due to increasing protons
80
New cards
s block metals
block with low first ionisation enthalpies due to few protons a limited outer shells
81
New cards
hydroxide
product of water and group 2 metal, also produces H2
82
New cards
oxides
product of when oxygen combines with a group 2 metal
83
New cards
strong alkaline solutions
when group 2 oxides react with water forming metal hydroxides which dissolve, expect magnesium oxide, increases down group
84
New cards
neutralise acids
both are bases so react with acids to form salts and water
85
New cards
group 2 hydroxide
solubility increases down group (single negative charge)
86
New cards
group 2 oxide
solubility decreases down group (double negative charge)
87
New cards
thermal decomposition
when heat is added to group 2 carbonates they form the oxide and CO2
88
New cards
thermal stability increases
change in thermal stability down group due to smaller cations (group 2) distorting the large carbonate more due to charge density
89
New cards
salts
neutral ionic compounds with a cations and anions
90
New cards
acids
substances with a pH less than 7
91
New cards
bases
substances with a pH more than 7
92
New cards
HN4+
ammonium
93
New cards
NO3-
nitrate
94
New cards
HCO3-
hydrogencarbonate
95
New cards
(SO4)2-
sulfate
96
New cards
(CO3)2-
carbonate
97
New cards
cation first
order when naming salts
98
New cards
most sulfates
soluble except barium, calcium, lead which form white ppt
99
New cards
lithium, sodium, potassium, ammonium and nitrates
all soluble salts
100
New cards
most chlorides, bromides, iodides
soluble salts except silver halides, copper iodide (white ppt), lead chloride and bromide (white ppt) and lead iodide (yellow ppt)