Bio Final test 1
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
All the organisms in your campus make a
Community
Systems biology is mainly an attempt to
understand the behavior of biological systems by studying interactions among its parts
A controlled experiment is one that
tests experimental and control groups in parallel
What best demonstrates the unity among all organisms
structure and function of DNA
What best distinguishes hypotheses from theories in science
Hypotheses usuallly are relatively narrow and theories have broad explanatory power
What is an example of qualitative data
The fish swam in a sigzag motion
What best describes the logic of scientific inquiry
If my hypothesis is correct, I can expect certain test results
In the term trace element, the adjective trace means that
the element is needed in small amounts
Compared with 31P, the radioactive isotope 32P has
one more neuron
The reactivity of an atom arises from
The existence of unpaired electrons in the valence shell
What is true of all atoms that are anions
The atom has more electrons than protons
What correctly describes any chemical reaction that has reached equilibrium
The rates of the forward and reverse reactions are equal
What represents the 18O isotope of oxygen
8p+, 10n0, 8e-
The atomic number for sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound
H2S
What coefficients must be placed in the blanks so that all atoms are accounted for in the products
C6H12O6 —> __ C2H60 + __ CO2
2;2
What is a hydrophobic material
Wax
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
Number of molecules
Measurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?
10-4 M
Measurements show that the pH of a particular lake is 4.0. What is the hydroxide ion concentration of the lake?
10-10 M
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50L container of cold water, what would be the approximate increase in the temperature of the water? (A liter of cold water weighs about 1 kg)
10 C
Organic chemistry is currently defined as
the study of carbon compounds
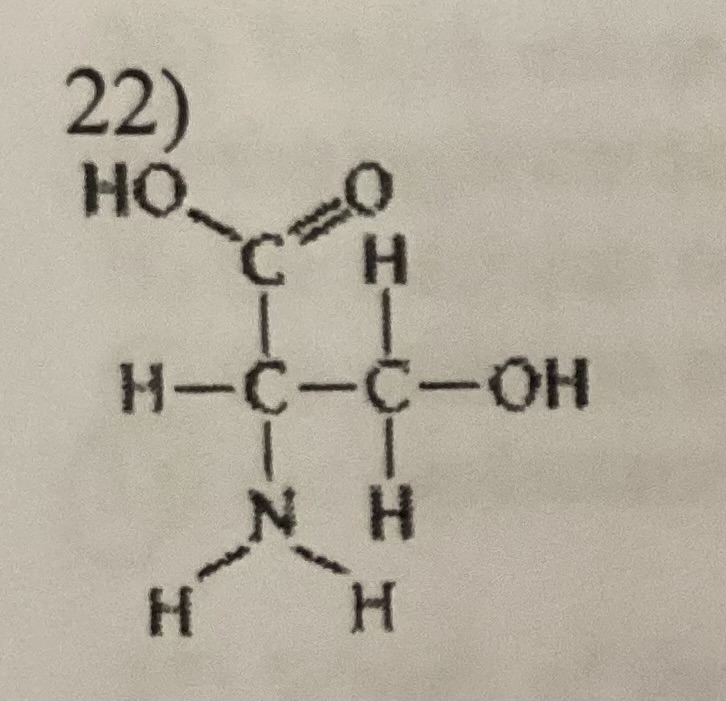
What functional group is not present in the molecule
sulfhydryl
What chemical group is most likely to be responsible for an organic molecule behaving as a base
amino
What hydrocarbon has a double bond in its carbon skeleton
C2H4
Enzymes that break down DNA catalyze the hydrolysis of the covalent bonds that join nucleotides together. What would happen to DNA molecules treated with these enzymes>
The phosphodiester linkages of teh polynucleotide backbone would be broken
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by linking ten glucose molecules together by dehydration reactions?
C60H102O51
Which of the following pairs of base sequences could form a short stretch of a normal double helix of DNA
5’ ATGC 3’ with 5’ GCAT 3’