Chemistry- Kinetics
0.0(0)
0.0(0)
Card Sorting
1/21
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
1
New cards
Rate Law for the Zero Order
Rate = k
2
New cards
Rate Law for the First Order
Rate = k[A]
3
New cards
Rate Law for the Second Order
Rate = k[A]^2
4
New cards
Integrated Rate Law for the Zero Order
[A] = -kt + [A]v0
![[A] = -kt + [A]v0](https://knowt-user-attachments.s3.amazonaws.com/aedfb904b40a4fb3934975b5ffb31f2c.jpeg)
5
New cards
Integrated Rate Law for the First Order
ln[A] = -kt + ln[A]v0
![ln[A] = -kt + ln[A]v0](https://knowt-user-attachments.s3.amazonaws.com/eba60cb68c484000a8478d04b5341770.jpeg)
6
New cards
Integrated Rate law for the Second Order
1/[A] = kt + 1/[A]v0
![1/[A] = kt + 1/[A]v0](https://knowt-user-attachments.s3.amazonaws.com/494aacaba2e040a2a999f9b252b8e7e6.jpeg)
7
New cards
Plot needed to give a straight line for the Zero Order
[A] versus t
8
New cards
Plot needed to give a straight line for the First Order
ln[A] versus t
9
New cards
Plot needed to give a straight line for the Second Order
1/[A] versus t
10
New cards
Relationship of rate constant to the slope of a straight line for the Zero Order
Slope = -k
11
New cards
Relationship of rate constant to the slope of a straight line for the First Order
Slope = -k
12
New cards
Relationship of rate constant to the slope of a straight line for the Second Order
Slope = k
13
New cards
Half-life for the Zero Order
tv1/2 = [A]v0/2k
![tv1/2 = [A]v0/2k](https://knowt-user-attachments.s3.amazonaws.com/fcf8d4c91a234cb9a3eaf0186a7a4872.jpeg)
14
New cards
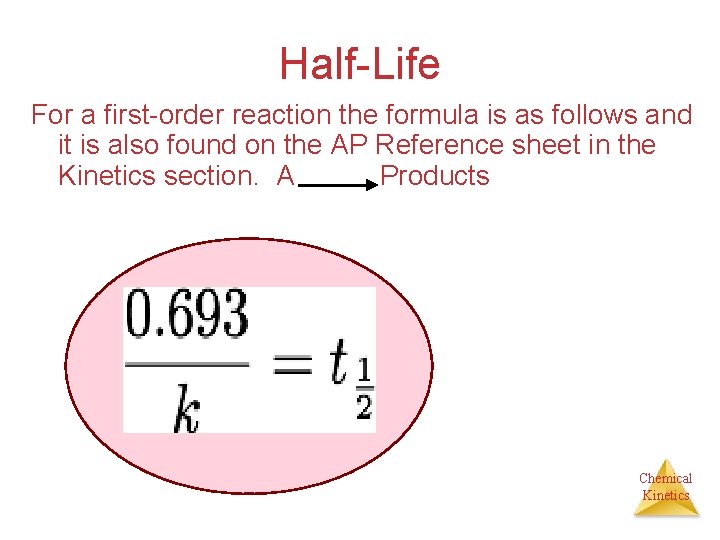
Half-life for the First Order
tv1/2 = 0.693/k
15
New cards
Half-life for the Second Order
tv1/2 = 1/k[A]v0
![tv1/2 = 1/k[A]v0](https://knowt-user-attachments.s3.amazonaws.com/96bfc14438274fb78d09d9eaca35de78.jpeg)
16
New cards
Activation Energy symbol
Eva
17
New cards
Universal Gas Constant symbol
R
18
New cards
Kelvin Temperature symbol
T
19
New cards
Fraction of collisions with energy Eva or greater at temperature T symbols
e^-Eva/RT
20
New cards
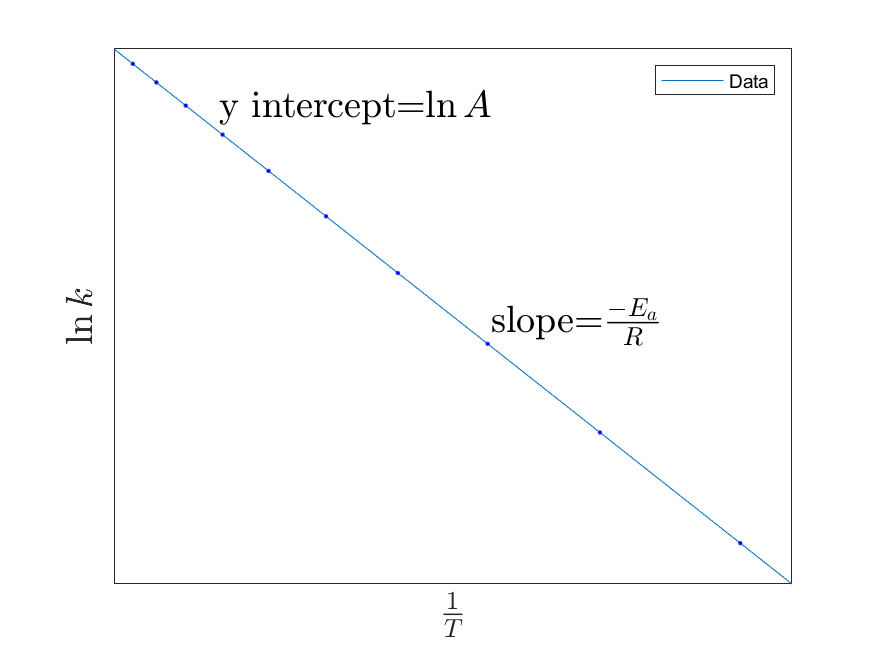
Value of Eva formula
Slope = -Eva/R
21
New cards
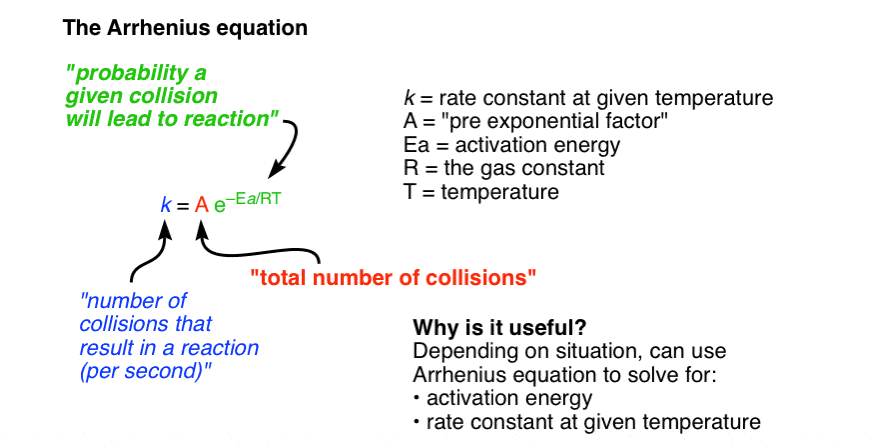
The Arrhenius Equation
k = Ae^-Eva/RT
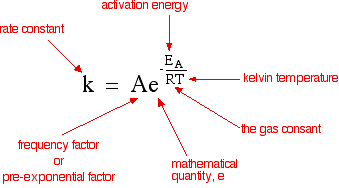
22
New cards
Frequency Factor symbol
A