MOD2 - Modern Atomic Theory
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
atom
Greek word “atomos” — indivisible
Smallest particle of matter
Law of conservation of mass
Law of constant composition
Law of multiple proportions
The three atomic theories by John Dalton
Conservation of Mass
Dalton’s Atomic Theory
by Antoine Lavoisier
Mass is neither created nor destroyed
Mass of product = Total mass of reactants
ex. 2H2 + O2 —> 2H2O
Constant Composition
Dalton’s Atomic Theory
aka “Law of Definite Composition”
Relative number of atoms per element in the compound is same in any sample
ex. Carbon dioxide in soda, dry ice, in blood
Multiple Proportions
Dalton’s
Elements A and B of compound A+B have respective ratios w/ small whole numbers
Different compounds from the same elements have different relative number of atoms
Cathode Ray Particles
The experiment that lead to the discovery of electrons.
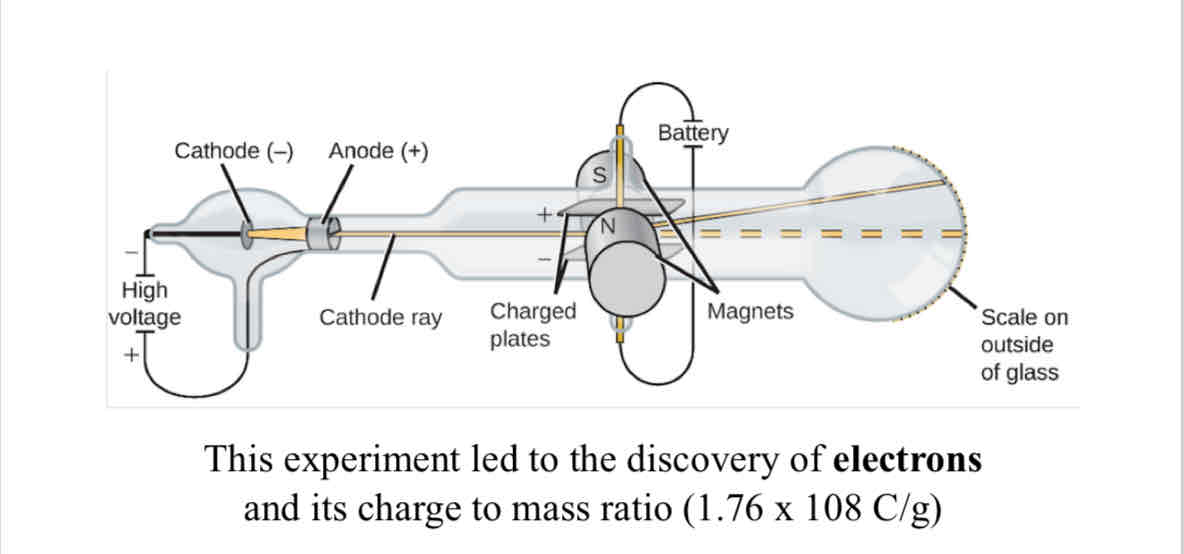
subatomic particles
Many discoveries led to the fact that the atom was made of even smaller particles called ______
ex. Electrons and cathode rays, radioactivity, nucleus, protons, and neutrons
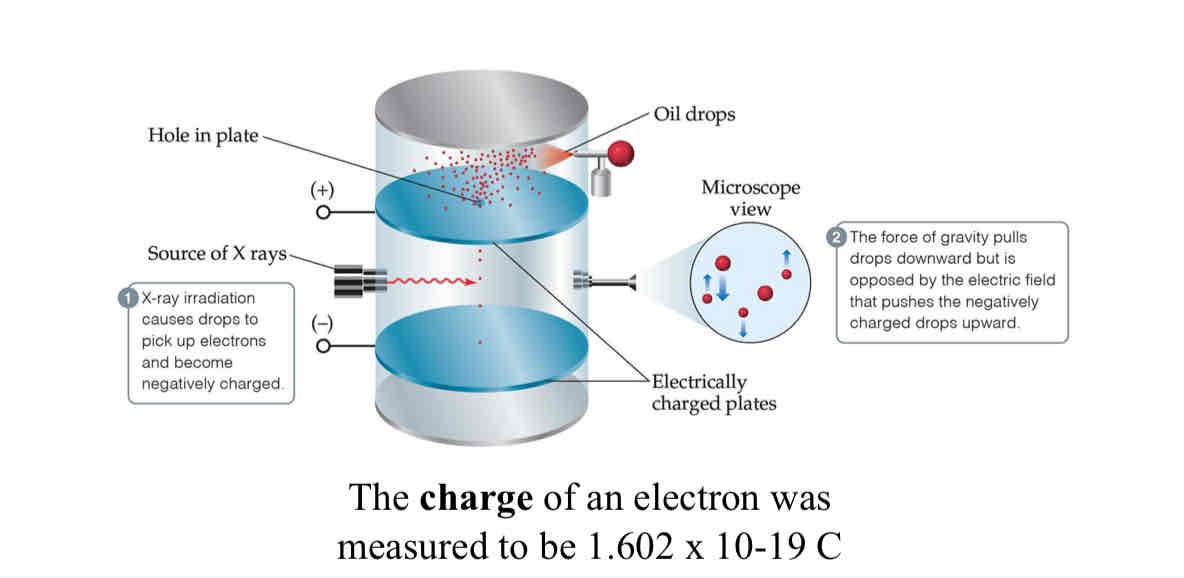
Millikan Oil-Drop Experiment
The experiment that lead to the discovery of electron charge.
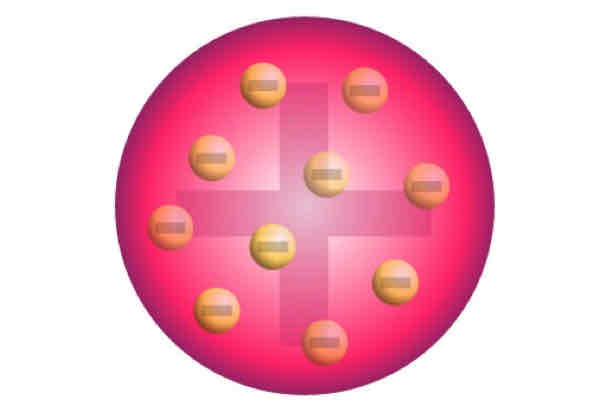
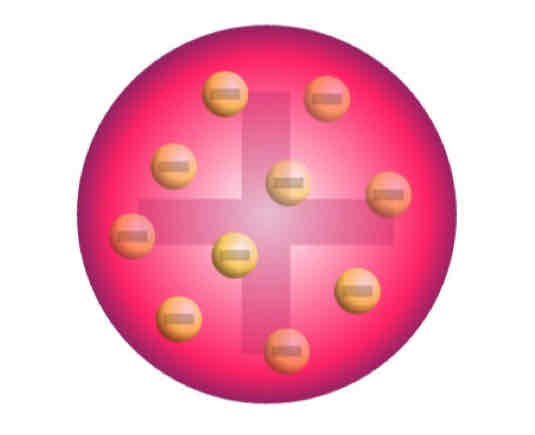
Thomson’s Model of the Atom
Model of the Atom
Electrons are scattered in a positively charged space, its mass evenly distributed
Rutherford’s Gold Foil Experiment
The experiment that lead to the discovery of the positively-charged nucleus, making Thomson’s model irrelevent.
The model violates the laws of physics.
The problem of Rutherford’s Nuclear Model of the Atom
electromagnetic radiation
Moves as waves through space at the speed of light
Each element is composed of extremely small particles called atoms.
Dalton’s Atomic Theory
Recite the postulate associated w/ the illustration
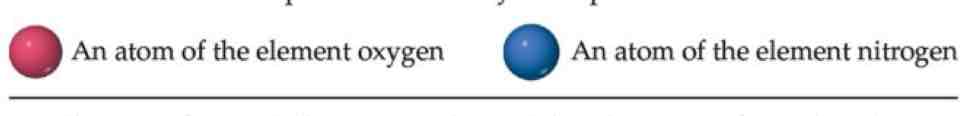
All atoms of a given element are identical, but the atoms of one element are different from the atoms of all other elements.
Dalton’s Atomic Theory
Recite the postulate associated w/ the illustration

Atoms of one element cannot be changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions.
Dalton’s Atomic Theory
Recite the postulate associated w/ the illustration

Compounds are formed when atoms of more than one element combine: a given compound always has the same relative number and kind of atoms.
Dalton’s Atomic Theory
Recite the postulate associated w/ the illustration
J.J. Thomson
He discovered the electron
Rutherford’s Nuclear Model of the Atom
Model of the Atom
wavelength (λ)
Distance between corresponding points on adjacent waves
frequency
Number of complete waves passing any point per second
waves
All electromagnetic radiation travels as _____.
3.00 × 108 m/s
Speed of light (c) = _____
c = λν
Speed of light formula
Max Planck
He provided the explanation for thr behavior of energy.
quanta
Behavior of Energy
Energy comes in packets called _____
This also explains the photoelectric effect
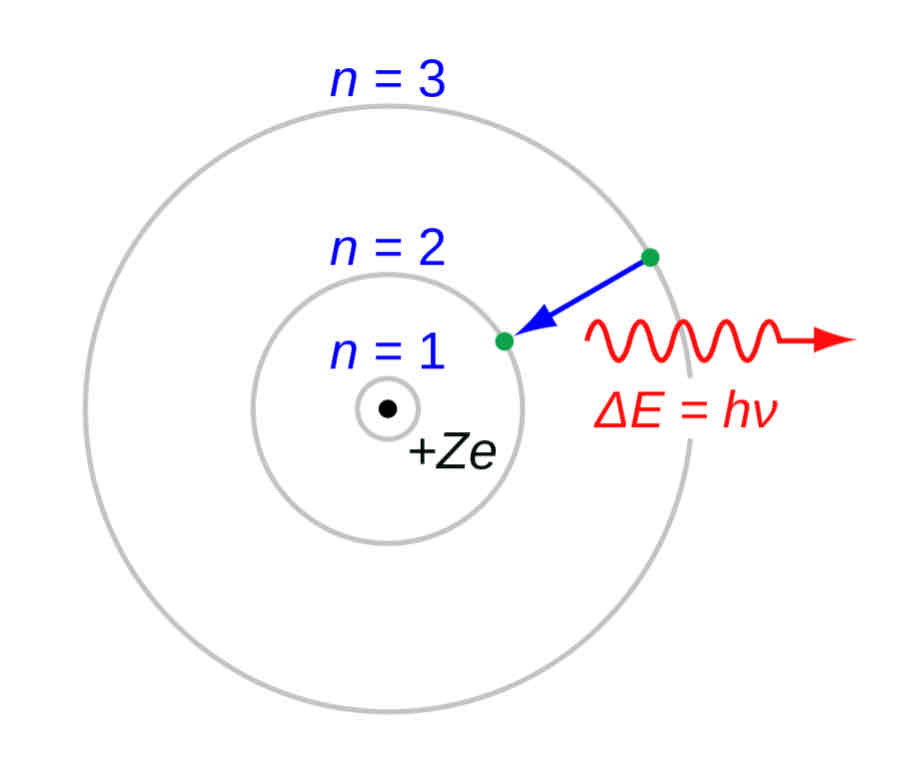
Bohr’s Model
Behavior of Energy
Energy of electrons are quantized
Electrons orbit around the nucleus at discrete radii
BUT this is only applicable to hydrogen
discrete—cannot have more or less
Meaning of quantized
Schrodinger’s Model
Behavior of Energy
aka Quantum Model
Used wave-particle duality to solve the behavior of atoms through equations called wave functions
Solutions of the equations gave probabilities
Widely accepted model today
2
Number of electrons that can fit in one orbital
Heisenberg’s Uncertainty Principle
The speed and position of the electron cannot be known simultaneously
Electrons (e-)
Negatively charged unit of atom
Charge of -1
Exact location outside nucleus unknown
Tinier than other subatomic particles
Mass is negligible
Proton (p+)
Subatomic Particles in the Nucleus
Positively charged unit of atom
Charge of +1
Does not move
Characterizes an element
Contributes to mass of atom
Neutron (n or n0)
Subatomic Particles in the Nucleus
Neutral unit of atom
Charge of 0
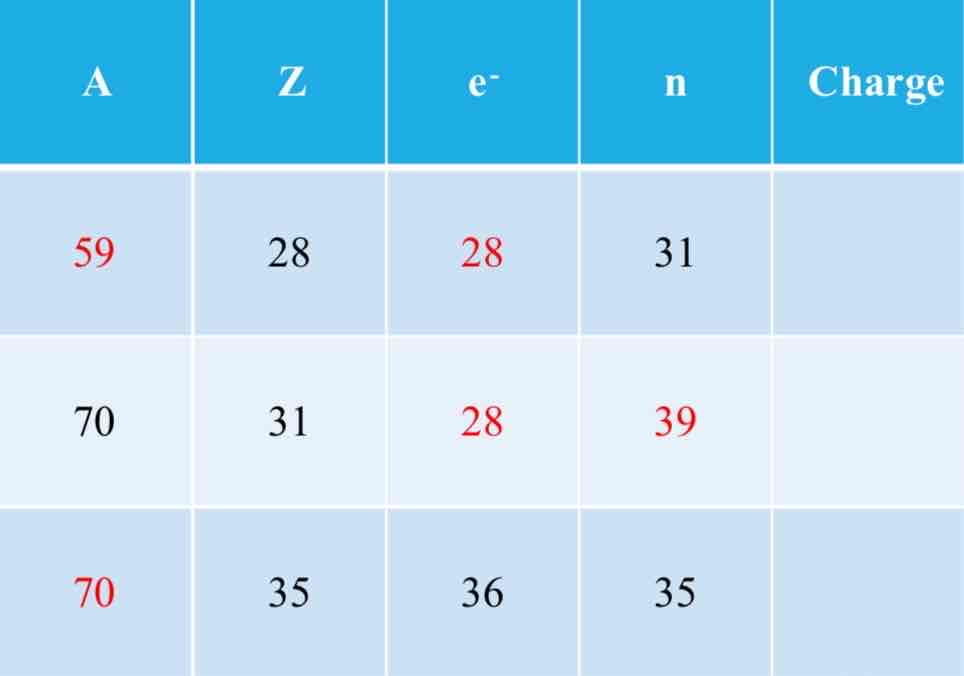
mass number; protons + neutrons
Atomic symbol A is called the ________ and indicates the number of _______.
atomic number; protons
Atomic symbol Z is called the ________ and indicates the number of _______.
protons (atomic number)
The number of electrons is equal to _____ unless it is an ion.
Isotopes
Atoms of an element with varying mass number
An atoms’s number of protons is always the same, but its number neutrons may vary
ex. Carbon 12 and Carbon 14
Ions
Charged atoms
Indicated by a loss or gain of electrons
Cation (+)
Atom with more protons than electrons
Excess positive charge
Anion (-)
Atom with more electrons than protons
Excess negative charge
0
+3
-1
Fill in the missing charges
boom in the discovery of elements; Triadic; Every eighth element
History of the Periodic Table
18th-19th centuries: ___________
Trends among elements:
_______ relationship according to atomic mass (ex. Li, Na, K)
__________ had similar properties (Law of Octaves)
First or first two letters of the element
O - Oxygen
Ca - Calcium
Latin name
Au - Gold (Aurum)
W - Tungsten (Wolfram)
Locations or people
The three bases for atomic symbol naming
amu - atomic mass unit
Unit of measurement of atomic weight
1 amu = 1.66054 × 10^-24
1 amu = _________
∑[(isotope mass)(percentage)]
Atomic weight formula for isotopes
Dmitri Mendeleev
Father of the periodic table
Organized the elements by atomic mass
Observed patterns while arranging elements
Group
Period
Periodic Table
Column: ______
Rows: ______
reactivity
reactive
Periodicity
Same group, same ______
Groups on the left are more _______
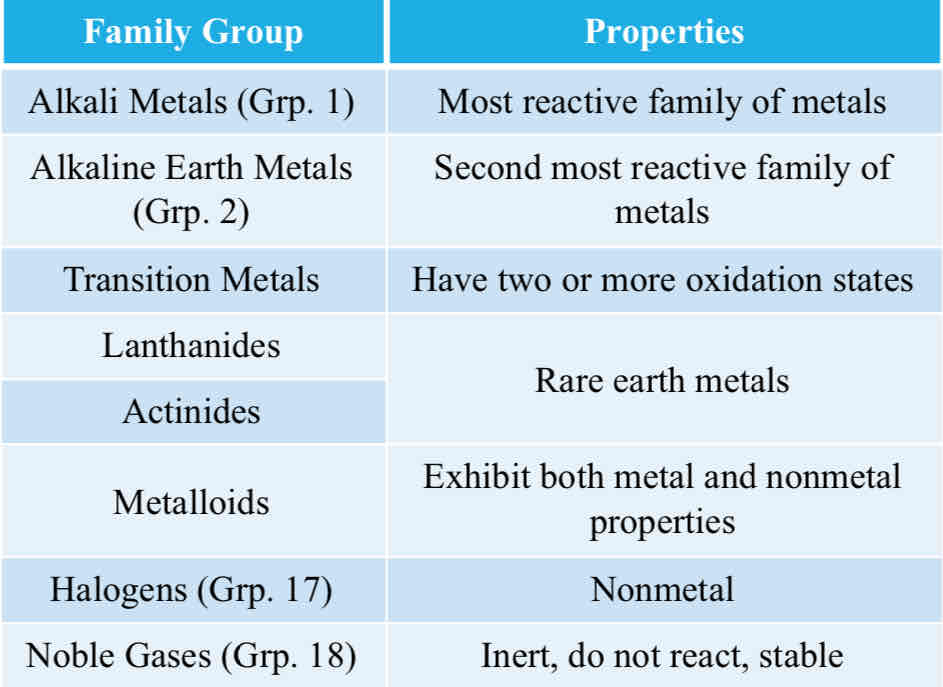
Family Groups (done)
Family Groups
Just for review, explain the table in your own terms.
Photoelectric effect
Each metal has its own energy at which it ejects electrons
Higher energy, more electrons emitted
Lower energy, electrons are not emitted
Energy is proportional to frequency: E = hν
Planck’s constant = 6.626 × 10^-34 J•s
Planck’s constant = __________
Ground state
Lowest energy level in an atom
Nearest to the nucleus
Most stable organization
Where electrons are when no energy has been absorbed
Excited state
Above ground state
Where electrons are when the atom absorbs a quantum of energy
light
Transition of electron from higher to lower energy levels releases the absorbed energy in the form of _____.
Energy levels
Orbitals
Shells: _______
s – 3 electrons ; n = period ; 1 orbital
p – 6 electrons ; n = period ; 3 orbitals
d – 10 electrons ; n = period ; 5 orbitals
f – 14 electrons ; n = period ; 7 orbitals
Orbitals
Subshells:
s – __ electrons ; n = __ ; __ orbital
p – __ electrons ; n = __ ; __ orbitals
d – __ electrons ; n = __ ; __ orbitals
f – __ electrons ; n = __ ; __ orbitals
Principal Quantum Number (n)
Quantum Numbers
aka the Period
Describes the energy level on which the orbital resides
Values are integers ≥ 1
Correspond to the values in the Bohr model
Angular Momentum Quantum Number (l)
Quantum Numbers
Defines the shape of the orbital.
Values are integers ranging from 0 to (n − 1)
Orbital group designates its different values
Defines the shape of the orbitals
Magnetic Quantum Number (ml)
Describes the three-dimensional orientation of the orbital
Allowed values are integers ranging from −l to l including 0
Where the last electron is located in Hund’s Rule
Magnetic Spin Quantum Number (ms)
Describes its magnetic field, which affects its energy
Reason why there are only two electrons allowed in an orbital
either -1/2 or +1/2
Which way the arrow faces in Hund’s Rule
-1/2 means arrow faces down
+1/2 means arrow faces up
the same energy level
the same sub shell
Energy Levels Multi-electron Atoms
Energies differ due to repulsion of electrons
Not all orbitals on ________ are degenerate: ns < np < nd < nf
Orbitals in ________ are degenerate
No two electrons in the same atom can have the same set of four quantum numbers (all electrons in an atom are unique)
Pauli Exclusion definition
Electron configuration
Arrangement of electrons in the orbitals of an atom
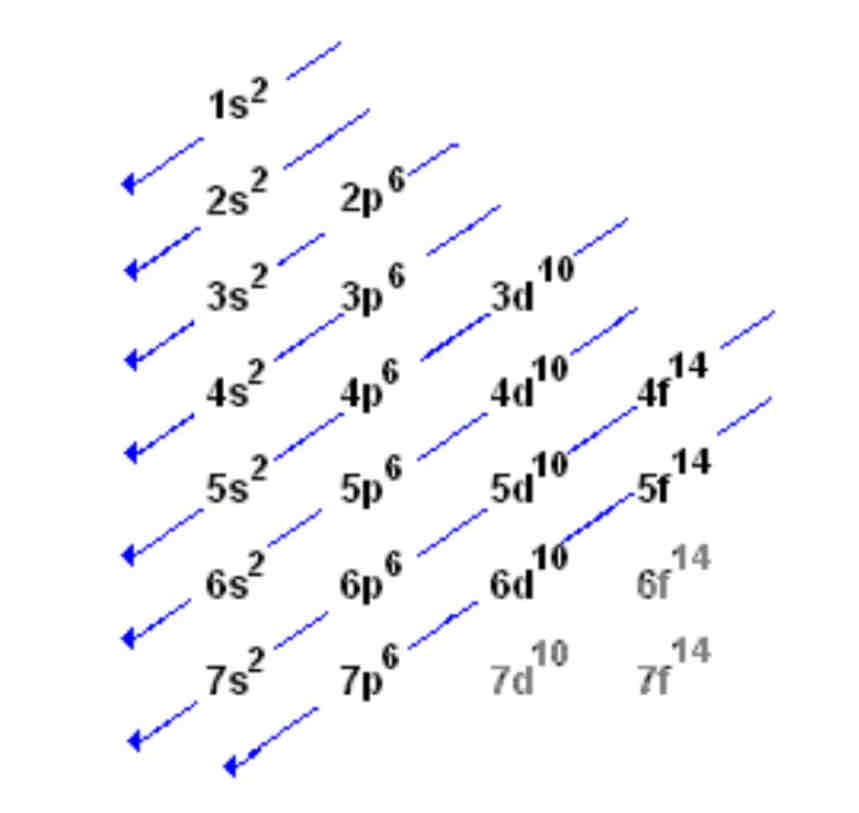
Aufbau Principle
Electrons fill the orbital with the lowest energy first
maximized
Hund’s Rule
For degenerate orbitals, the lowest energy is attained when the number of electrons having the same spin is _______.
Valence electrons
Electrons in the outermost shell
Core electrons
electrons in the remaining shells (electrons that aren’t the valence electrons)
Effective Nuclear Charge
Net electric field created by the nucleus and the electron density of the other electrons
Simultaneous attraction of electrons to the nucleus and repulsion among electrons
Smaller than the actual charge (Z)
right; down
Effective Nuclear Charge Trend:
inreases to the ______ of a period & ______ a group
Zeff = Z-S
Effective nuclear charge equation
As more electrons fill the orbital, the protons pull the electrons inward more, causing a decrease in the radius.
Explanation of atomic radius
to the bottom left
Atomic Radius Trend
smaller
Because the outermost electron is removed and repulsions between electrons are reduced
Ionic Radius
Cations are _____ than their parent atoms.
bigger
Because electrons are added and repulsions between electrons are increased
Ionic Radius
Anions are ____ than their parent atoms.
Ionization Energy
* More electrons in a subshell = Higher ionization energy = HARDER to remove an electron
Minimum energy required to be absorbed by atom to release an electron from the ground state of a gaseous atom or ion
to the top right
Ionization Energy Trend
to the top right
Ionization Energy Trend
First ionization energy
Energy required to remove the first electron
Second ionization energy
Energy required to remove the second electron
Electron Affinity
Energy change accompanying the addition of an electron to a gaseous atom
Exothermic in nature
Cl + e- → Cl-
2A - s is full
5A - p is half full
8A - p is full
* Electron affinity value for these groups are positive
Groups excluded in electron affinity because their last subshell is full or half full (stable sila)
Cation
Metals tend to form this ion
Anion
Nonmetals tend to form this ion
Because they have the same number of valence electrons
Reason why elements in the same group have similar properties