History of the atomic model
0.0(0)
Card Sorting
1/27
Earn XP
Last updated 7:01 AM on 11/11/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
1
New cards
Who was the first scientist to theorize the atom?
Democritis
2
New cards
When did Democritus live?
460-370 BCE (5th century BCE)
3
New cards
What did Democritus theorize?
He believed that matter was made up of tiny particles that exist in empty space. His idea was based on reason and logic
4
New cards
Who believed matter was a combination of the four elements, earth, fire, air, and water?
Aristotle
5
New cards
When did Aristotle live?
384-322 BCE (3rd century BCE)
6
New cards
How did Aristotle disagree with Democritus?
Aristotle did not believe atoms existed. He thought that empty space couldn't exist
7
New cards
Who was John Dalton?
The first scientist to conduct experiments.
8
New cards
What did Daltons experiments show?
From his experiments, he got evidence of chemical reactions and changes in mass
9
New cards
When did Dalton live?
1766-1844
10
New cards
What are the five points of Daltons theory of atoms?
1. Elements are made of tiny particles called atoms
2. All atoms of a given element are identical
3. The atoms of a given element are different from those of any other element
4. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms
5. Atoms are indivisible in chemical processes. Basically saying atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way the atoms are grouped together
2. All atoms of a given element are identical
3. The atoms of a given element are different from those of any other element
4. Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms
5. Atoms are indivisible in chemical processes. Basically saying atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way the atoms are grouped together
11
New cards
What did dalton think an atom looked like?
A solid ball

12
New cards
Who discovered the electron?
J.J. Thomson
13
New cards
When did Thomson live?
1856-1940
14
New cards
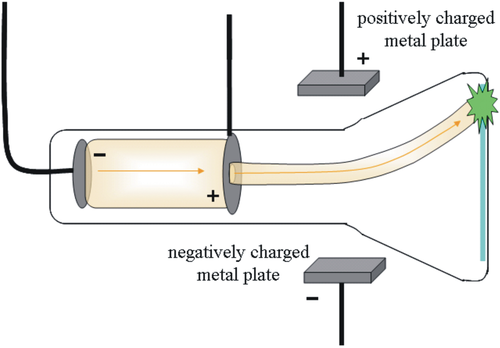
How did Thomson discover the electron?
Using cathode ray tubes
15
New cards
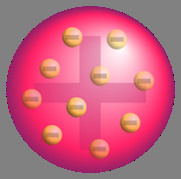
What did Thomson think an atom was?
A positively charged ball with negatively charged particles embedded inside
16
New cards
What are some of the names people call Thomson's model of the atom?
Plum pudding model, chocolate chip cookie model, raisin bun model
17
New cards
Who discovered the nucleus, theorized neutrons and invented the word proton?
Ernest Rutherford
18
New cards
When did Rutherford live?
1871-1937
19
New cards
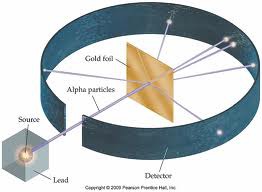
How did Rutherford discover the nucleus?
By shooting alpha particles through a sheet of gold foil. He found that most of the particles went through, showing there had to be empty space, but some bounced back showing there had to be a positively charged nucleus to reflect the particles.
20
New cards
What did Rutherford think the atom looked like?
He only theorized the neutron

21
New cards
What did James Chadwick discover?
The neutron
22
New cards
When did Chadwick live?
1891-1974
23
New cards
What types of experiments did Chadwick do?
Experiments using radioactive decay
24
New cards
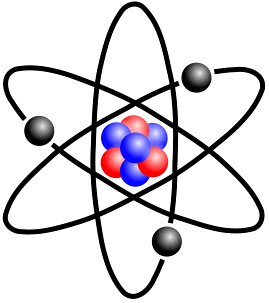
What did Chadwick think an atom looked like?
Similar to Rutherford
25
New cards
Who discovered electrons surround the nucleus in distinct energy levels?
Neils Bohr
26
New cards
When did Bohr live?
1885-1962
27
New cards
How did Bohr make his discovery?
He produced a line spectra of gases by passing them through electricity
28
New cards
What does a Bohr diagram look like?
