science quiz
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Compounds are made up of
2 or more elements chemically combined
Ex-salt (NaCl) water (H2O)
Elements are made up of only
One type of atom
Ex- anything on the periodic table
Homogenous mixtures
Have an appearance described as the same throughout
Ex- salt water tea
Heterogenous mixtures
Have an appearance described as not uniform (the same) throughout
Ex- pizza Salad
Solute
The substance that dissolves
dissolve
Mixing of a substance in liquid
Solution
The homogenous mixture formed when a substance dissolves in it
Solvent
Substance that dissolves the solute and is present in the greatest amount, usually a liquid
Physical property
A characteristics of a substance that can be observed or measured without changing the identity of a substance
Physical changes include
Melting/freezing bending crumbling/slicing evaporating and dissolving
Chemical changes include
Rusting heat/temp flames/flammability rotting reacting
Physical property and chemical property include
Physical property- density color and boiling point
Chemical property – flammability and reacting with water
Chemical property
A chemical property describes the ability of a substance to undergo a specific chemical change or reaction
Evidence that a chemical change has occurred
Production of a gas odor light or a change in color or temperature or the formation of precipitate, which is when you get a solid
pH scale
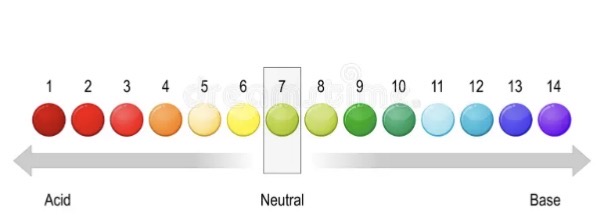
Physical change
Required to change pure substances like elements or compounds in a mixtures
Chemical change
Required to change elements into compound or compounds in elements