Principles of Toxicology Exam 2
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
117 Terms
Biotransformation (Metabolism in ADME)
enzymatic process of chemical modification that generally changes the physiochemical properties of a xenobiotic from those that favor absorption and distribution, to those that favor elimination (i.e., lipophilic → hydrophilic)
What is the exception to our definition of biotransformation?
elimination of volatile compounds by exhalation
Parent Xenobiotic
form that is absorbed and may be a substrate for biotransformation
Ultimate Xenobiotic
form that causes a change in the body, may be a product of biotransformation
Can the ultimate xenobiotic also be the parent xenobiotic?
Yes it can
What does TPSA measure?
the number of oxygen and nitrogen atoms that bind water by functioning as hydrogen
donors or acceptors
What is the relationship between passive diffusion and TPSA? (increasing/decreasing)
Rate of passive diffusion decreases with increasing TPSA
What value of TPSA restricts passive diffusion? (acids)
> 75 Angstrom
What value of TPSA restricts passive diffusion? (bases)
> 100 Angstrom
What is the process of xenobiotic biotransformation?
the process of converting lipophilic chemicals which are
readily absorbed from the GI and other sites into hydrophilic chemicals, which are readily excreted in
urine or bile
Hydrolysis Reactions
break covalent bonds in substrates
Reduction Reactions
transfer electron(s) to substrates
Oxidation Reactions
Remove electron(s) from substrate
Conjugation Reactions
Covalent attachment of chemical groups to substrate. Examples include: sulfonation, acetylation , methylation, conjugation with AA, etc.
Hydrolysis, reduction and oxidation expose or introduce a _____________?
functional group that can be converted into a water-soluble cojugate
(T/F): Phase I reactions always happen first
False; not always
What reaction(s) cause a modest increase in water solubility?
Oxidation, reduction, hydrolysis, and acetylation
What reaction(s) cause a modest decrease in water solubility?
methylation
What reaction(s) cause a marked increase in water solubility?
glucuronidation, sulfonation, glutathionylation and AA conjugation
What Log P and TPSA values are required for a drug to be absorbed by passive diffusion in the GI lumen?
Log P: 0-5
TPSA: <100 Angstrom
What range of Log P values are generally insoluble in the GI lumen?
Log P >5
What range of Log P values are absorbed orally in substrate for uptake transporter?
Log P < 0
What is activation?
conversion of a benign parent xenobiotic to a biologically active (ultimate) form, or increase in toxicity of parent xenobiotic
What is inactivation?
conversion of a biologically active xenobiotic to a benign or less toxic form (detoxification)
What organ expresses the largest number and (generally) the highest concentrations of xenobiotic- metabolizing enzymes?
The liver
What do efflux transporters do in the liver?
remove xenobiotics from blood and discharge xenobiotics into bile, or discharge metabolites into blood for excretion in urine
How do efflux transporters affect cells in the small intestine?
cells express high levels of certain enzymes
UM
ulta-rapid metabolizer
EM
extensive metabolizer (normal)
IM
intermediate metabolizer
PM
poor metabolizer
T/F: individual xenobiotic-transforming enzymes are located in a single organelle
True
What two factors influence biotransformation rates?
disease state & cofactor levels
T/F: Xenobiotic transformation is accomplished by a limited number of enzymes with broad substrate specificities
True
How are enzymes/substrates named in toxicology?
The broad spectrum of substrates prevents enzymes from being named after the reaction they catalyze, but rather
in families and subfamilies, then named according to primary AA sequence
What is distribution?
the systemic movement of xenobiotics within the body from the site of absorption to the target tissue (requires circulatory system)
What factors affect the rate of distribution? (4)
Blood flow
Sequestration in blood or off-target tissue
Transport from organ capillary beds into organ interstitial fluid
Affinity for target tissue
What is Vd?
Volume of distribution, apparent volume in which the amount of chemical disperses in order to produce the observed blood concentration
What is the equation for Vd?
Vd = (amount of drug in the body)/(blood concentration of drug)

What do low and high Vd values suggest?
Low Vd: chemical distributes only to the plasma (no tissue distribution)
High Vd: chemical distributes to the total body of water
T/F: storage/sequestration can not hinder distribution
False, xenobiotics may have an affinity for tissue other than their target organ of toxicity
What are the four (4) major sites of xenobiotic sequestration?
blood plasma
body fat
keratinized tissue/hair
bone
What is a “storage depot”?
a compartment where chemical is concentrated, but not major site of toxicity
T/F: binding of a xenobiotic to its receptor is reversible (in blood plasma)
True
What is the ratio of affinity?
koff / kon
What is kd?
equilibrium dissociation constant for a given xenobiotic ligand (L) and its receptor (R), a quantitative measure of binding affinity, xenobiotic concentration where 50% of binding sites are bound at equilibrium
Give the equation for kd
Kd = [L][R]/[LR]
What do low and high values of kd mean for affinity?
low kd = high affinity
high kd = low affinity
What are adipocytes?
Adipocytes are fat storing cells in adipose tissue
Which is more likely to be sequestered in body fat: highly or barely lipophilic xenobiotics?
Highly lipophilic xenobiotics (LogP > 1) are more likely to be sequestered (bioaccumulate) in body fat
Which two (2) organs have a high capacity for acting as storage depots?
liver and kidneys
What are osteoclasts?
bone breakdown via osteolysis, can release xenobiotics
What are osteoblasts?
bone formation via mineralization
T/F: the blood-brain-barrier is very effective against xenobiotic penetration
True
How does lipophilicity and ionization/polarization affect distribution of xenobiotics into the brain?
distribution increases as lipophilicity increases; distribution decreases as ionization decreases
What are the 3 primary routes of elimination in the body?
kidneys (urine), feces, lungs (exhalation)
What are nephrons and where are they located?
Nephrons are the functional units of the kidneys where urine formation occurs
What is the glomerulus in the nephrons?
Glomerulus: specialized capillary structure that serves as a size/charge filter due to its fenestrated (highly porous) endothelium
What does the tubule system do in nephrons?
Tubule system: modifies and concentrates the urine
What is a hydrolysis reaction?
hydrolysis: cleavage of covalent bonds by reaction with water
What are the 4 catalysts for hydrolysis reactions?
carboxylesterases, cholinesterases, paraoxoneses, and albumin
Does CES1 prefer to hydrolyze xenobiotics with small or large alcohol leaving groups?
small
Does CES2 prefer to hydrolyze xenobiotics with small or large alcohol leaving groups?
large
What is the difference between AChe and BChe?
AChe is highly selective to acetylcholine and has little to no role in metabolism while BChe hydrolyzes numerous xenobiotics like aspirin, heroine, cocaine, succinylcholine, etc.
_________ catalyzes the hydrolysis of many organophosphates, organophosphinites, aromatic carboxylic acid esters, cyclic carbonates, lactones and oxidized phosphates
paraoxonases (lactonases)
What are the 2 key features of paraoxonases?
contain R-SH group
calcium dependent
What are carboxylesterases and what organs/tissues are they found in (3)?
predominately microsomal enzymes present in liver, intestine, kidneys, other tissues
What 3 substances do carboxylesterases commonly hydrolyze?
aspirin, cocaine, heroin
What is a prodrug and what type of reaction activates it?
an inactive form of a medication that requires metabolic conversion within the body to become pharmacologically active; hydrolysis via carboxylesterases or cholinesterases
In an oxidation reaction, would an aldehyde (CHO) become an alcohol (OH) or carboxylic acid (COOH)?
carboxylic acid (COOH)
In a reduction reaction, would an aldehyde (CHO) become an alcohol (OH) or carboxylic acid (COOH)?
alcohol (OH)
What are cofactors?
molecules or ions that associate with some enzymes and are used to perform reactions
What are prosthetic groups?
a type of cofactor that are tightly bound by enzymes and are essential for enzyme function
Are NAD+/NADH and NADP+/NADPH cofactors or prosthetic groups?
cofactors
Are FAD/FADH2 and heme group/iron cofactors or prosthetic groups?
prosthetic groups
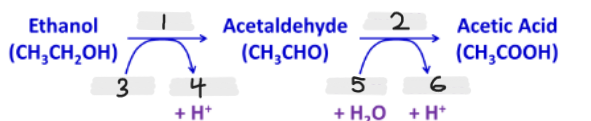
Label the missing parts of this image
ADH
ALDH
NAD+
NADH
NAD+
NADH
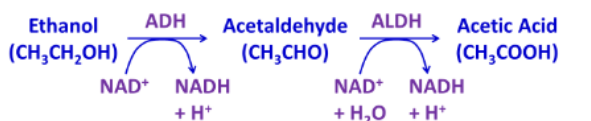
Which is found in the cytoplasm: ADH or ALDH?
ADH
Which is found in the mitochondria: ADH or ALDH?
ALDH
What is monooxygenation?
one atom from O 2 is incorporated into a substrate and the other is reduced to water
What organelle in which organ is the highest level of CYP observed?
liver smooth ER (present in virtually all tissues though)
What are the 5 requirements of the CYP monooxygenation reaction?
O2
Heme (Fe 2+/3+)
NADPH
NADPH - cytochrome P450 reductase
cytochrome b5
List all the primary Phase II Conjugation Reactions (6)
Methylation
N-Acetylation
Glucuronidation
Sulfonation
Glutathione
Amino acids
What is/are the required enzyme(s) for N-Acetylation reactions?
2 N-acetyltransferases (NATs); NAT-1 and NAT-2
What cofactor is required for the enzymes in N-Acetylation?
acetyl-coenzyme A (acetyl-CoA)
What are the two functional groups that act as substrates in N-Acetylation reactions?
amine (R-NH2) and hydrazine (R-NH-NH2)
What is the typical structure of R in a N-Acetylation reaction substrate?
R is usually an aromatic ring structure
Acetyl-CoA _______ the _______ group to the N-acetylation reaction
donates; acetyl
Describe the 2 steps of a N-Acetylation reaction
Step 1: Acetyl group is covalently attached to the enzyme
Step 2: Acetyl group is transferred to the xenobiotic amine/hydrazine functional groups
What type of Phase II reaction is this?
N-Acetylation
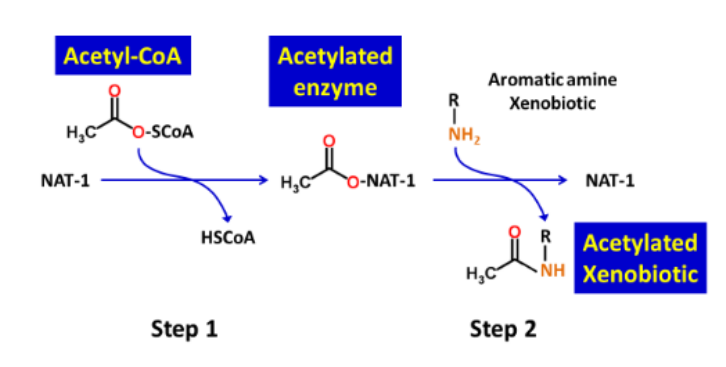
What type of Phase II reaction is this?
N-Acetylation
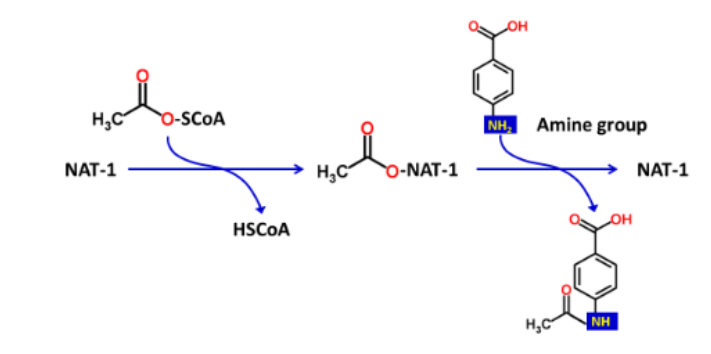
What type of Phase II reaction is this?
N-Acetylation
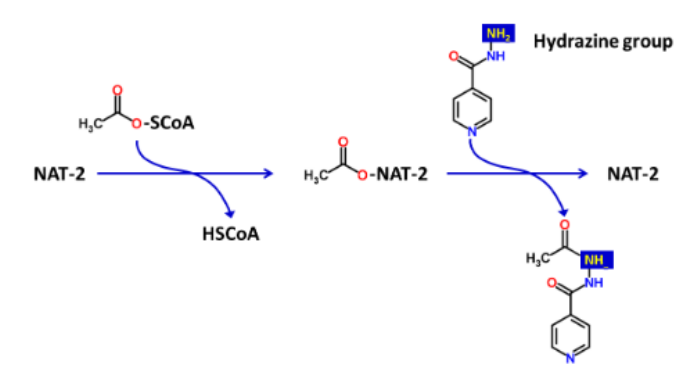
What type of Phase II reaction is this?
N-Acetylation
What is/are the required enzyme(s) for methylation reactions?
O-, N-, S- methyltransferases
What is the cofactor for methylation?
S-adenosylmethionine (SAM)
S-adenosylmethionine (SAM) _______ a _______ group to the xenobiotic substrate
donates; methyl
What are COMT and POMT?
COMT = catechol O-methyltransferase with 2 OH groups on a benzene ring
POMT = phenol O-methyltransferase with 1 OH group on a benzene ring
T/F: methylation represents an important pathway of detoxication
True
Does a methyl group increase or decrease lipophilicity of xenobiotic substrates?
increase lipophilicity
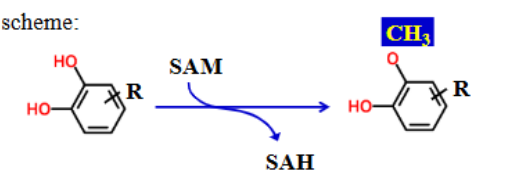
COMT or POMT?
COMT