OCR A M2 foundations in chemistry
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
Define an isotope
Atoms of the same element with different numbers of neutrons but the same number of protons and electrons
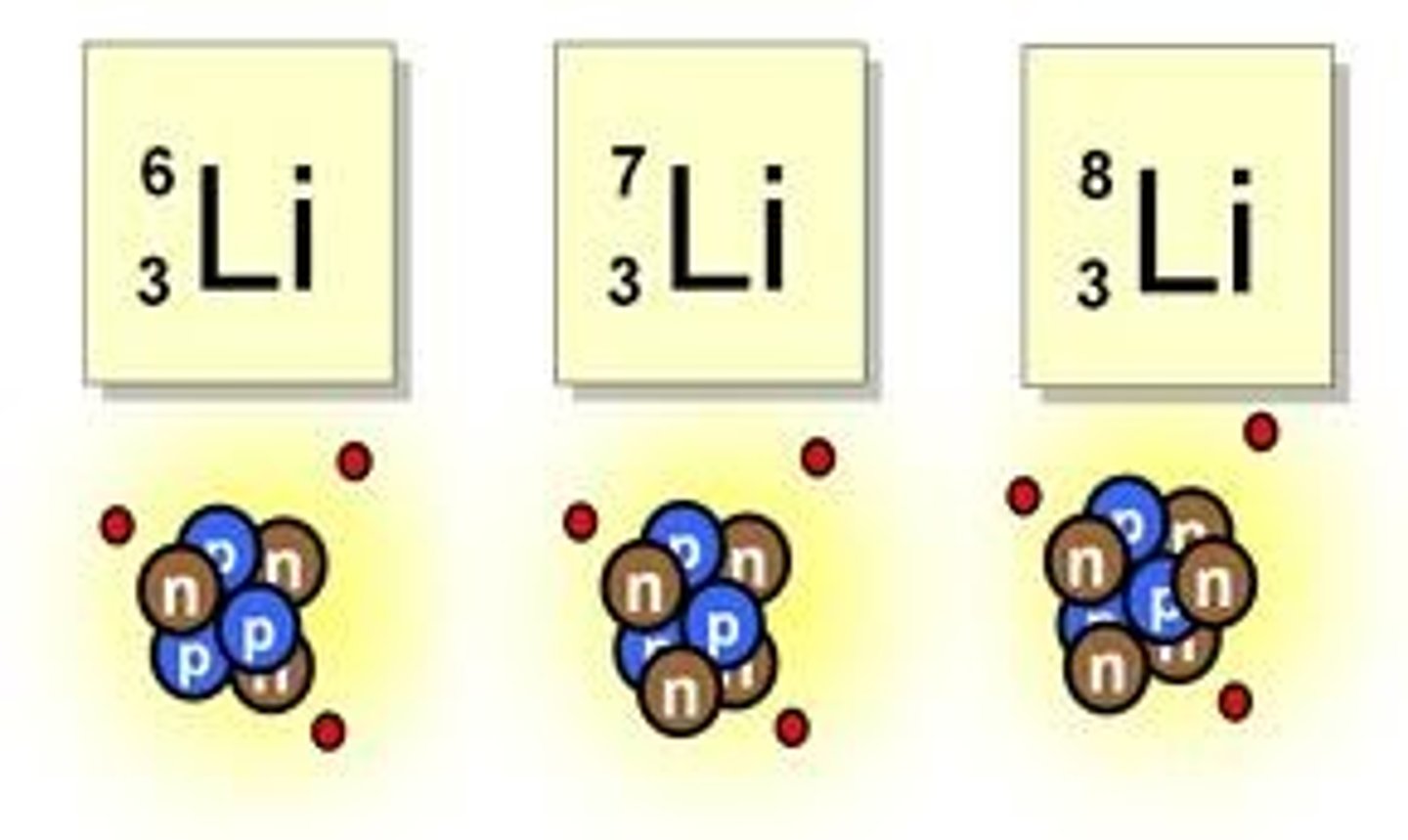
what does the term "relative isotopic mass" mean?
the mass of an isotope relative to 1/12th of the mass of an atom of Carbon-12
what does the term "relative atomic mass" mean?
is the weighted mean mass of an atom of an element relative to 1/12th of the mass of an atom of carbon-12
what does the term "relative molecular mass" mean?
is the weighted mean mass of a molecule relative to 1/12th of the mass of an atom of carbon-12
What is the atomic number
number of protons
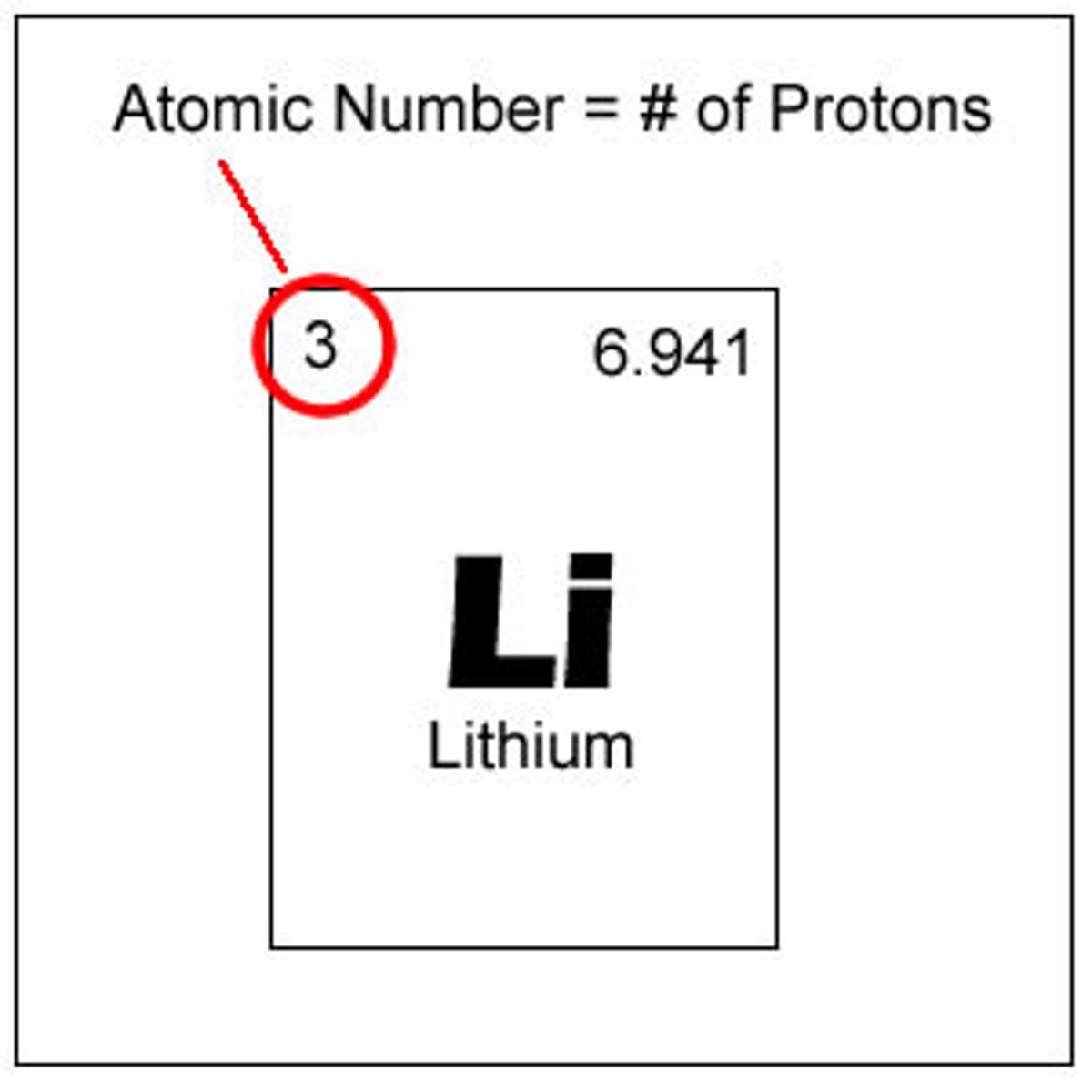
what is the atomic mass
Number of protons and neutrons
define "the mole"
one mole is the amount of substance that contains 6.02x10^23 particles
state the formula linking moles, mass and molecular mass
moles = mass divided by molecular mass
what are the charges of: Al, Ag, and Zn
Al= 3+
Ag= +
Zn= 2+
Charges and masses of protons, neutrons, and electrons
PROTON CHARGE= +1
ELECTRON CHARGE= -1
NEUTRON CHARGE= 0
PROTON RELATIVE MASS= 1
ELECTRON RELATIVE MASS= 1/2000
NEUTRON RELATIVE MASS= 1
Name all of the diatomic elements
Bromine, Iodine, Nitrogen, Chlorine, Hydrogen, Oxygen, Fluorine
What is Avogadro's constant?
6.02 x 10^23 mol the number of particles in each mole. to find number of atoms, fine number of moles and x by constant (same for molecules but using Mr of whole molecule to find moles)

state the equation for percentage yield?
percentage yield = actual yield/theoretical yield x 100
yield could be in moles {note: when working backwards, divide 100 by the theoretical mass, not the theoretical mass by 100)
state the equation for atom economy
mass of desired product/total mass of reactants x 100
State the ideal gas equation
PV = nRT
p= pressure (Pa)
V= volume (m^3)
n= amount of substance (mol)
R= ideal gass equation (8.314)
T= temperature (k)
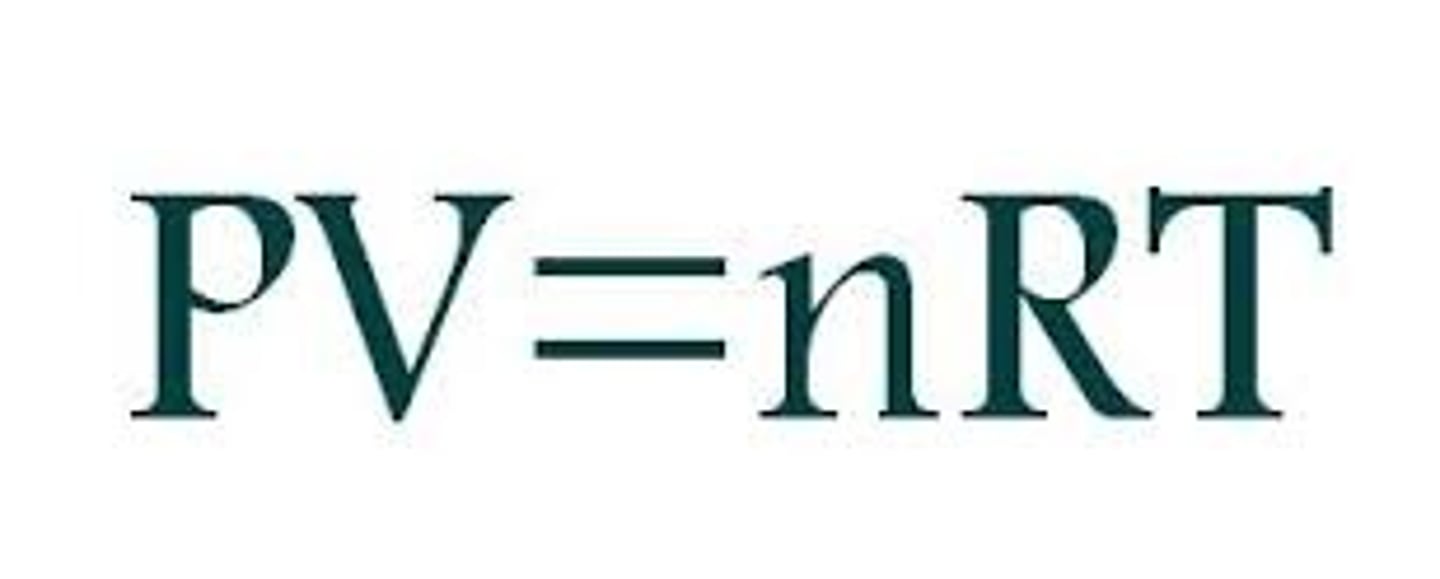
how do you convert from Kpa to Pa
x 1000
how do you convert from dm^3 to m^3?
divide by 1000
how do you convert from *c to kelvin
+ 273
what is water of crystallisation
crystals of a compound contain water molecules bonded to the crystal structure

what is the symbol that represents water of crystallisation?
.
what does the term "anhydrous" mean?
does not contain water of crystallisation
what are the problems with water of crystallisation?
1) if value is less then expected, not all water molecules where driven off by heating
2) if value is more than expected, the compound has further decomposed
define "atomic orbital"
A region around the nucleus that can hold up to two electrons with opposite spins [s orbital = spherical and p orbital = dumb-bell]
![<p>A region around the nucleus that can hold up to two electrons with opposite spins [s orbital = spherical and p orbital = dumb-bell]</p>](https://knowt-user-attachments.s3.amazonaws.com/5ad44365-f08b-457e-af80-fbc94fdee5a5.jpg)
which 2 elements are exceptions in electron configuration and why?
chromium and copper
their 3d subshells are more stable when half or completely full [s orbital = spherical and p orbital = dumb-bell]
describe the relative energies of s and p orbitals
p-orbitals have greater energy than s-orbitals.
p-orbitals have equal energies to eachother
how to work out number of electrons is a specific shell
times the shell number by 8
difference between sub shell, orbital and shell
sub-shell = eg: 1s sub shell has 2 electrons
orbital = eg: the 3p orbital has 6 electrons
shell = eg; the 3rd shell has a total of 18 electrons (3s has 2, 3p has 6 and 3d has 10 so total of 18)
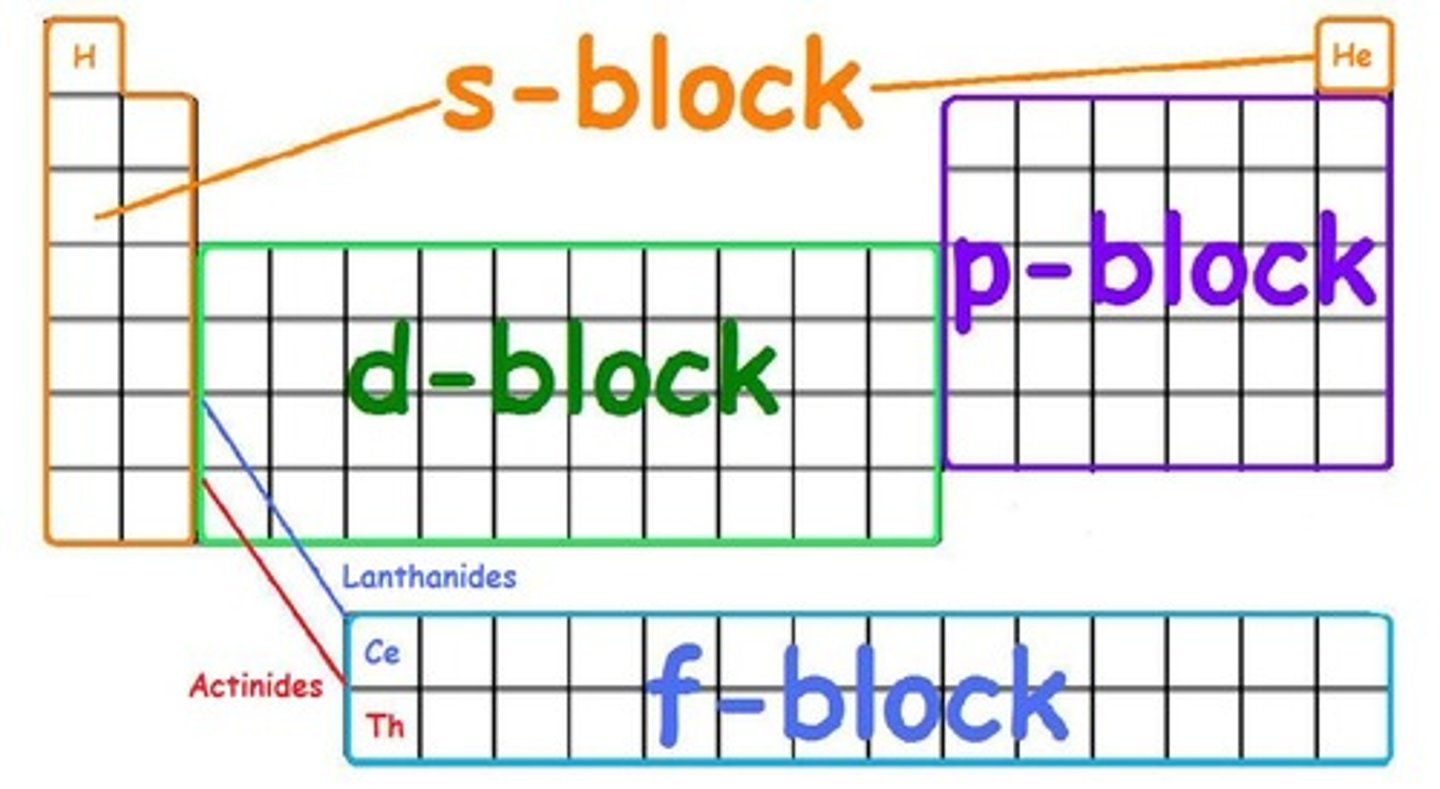
can you name and locate the 4 blocks in the periodic table?
state the rules when filling orbitals
orbitals with the lowest energy are filled first.
can only have 2 electrons in the same orbital with opposite spins.
4s fills before 3d
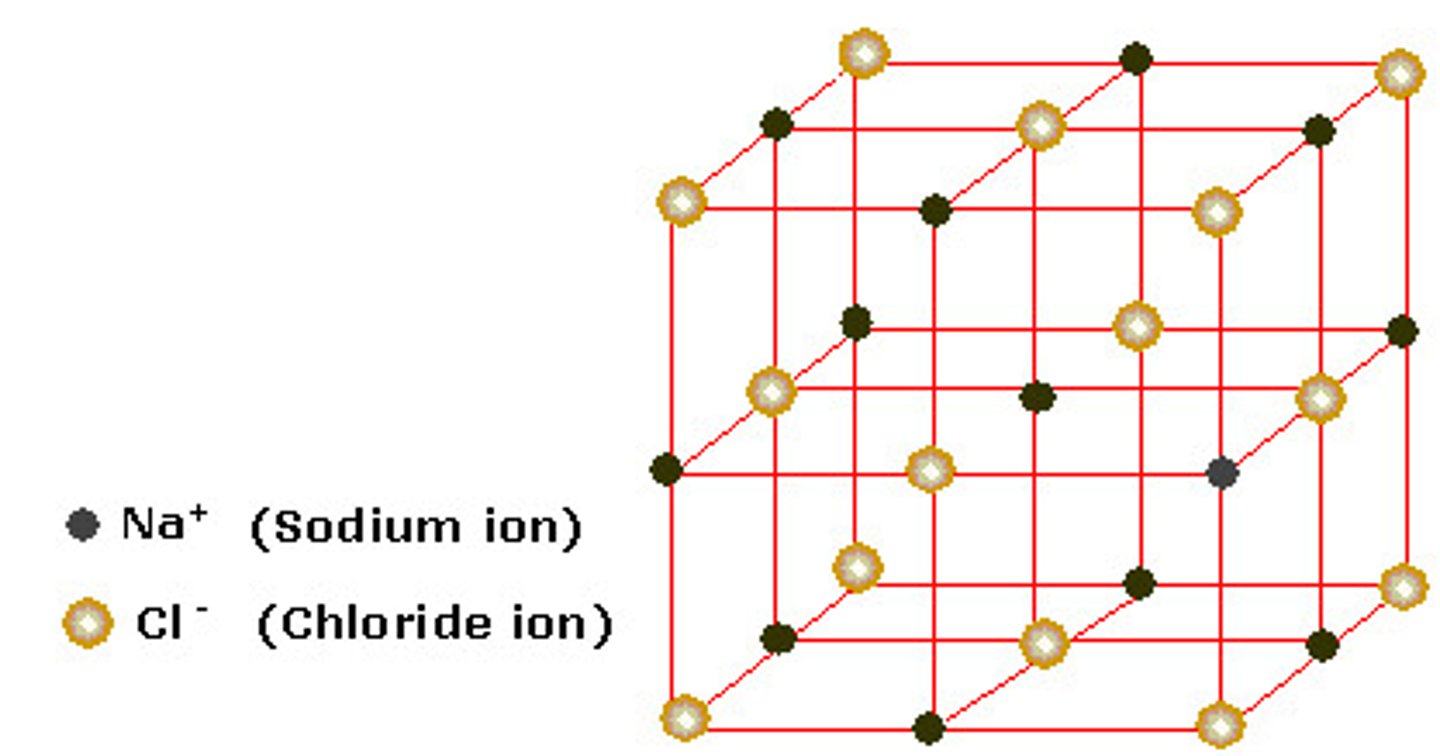
define ionic bonding
The electrostatic attraction between oppositely charged ions
what is the structure of ionic compounds?
each ion in an ionic compound is surrounded by oppositely charged ions that are strongly attracted in all directions, forming a giant ionic lattice.
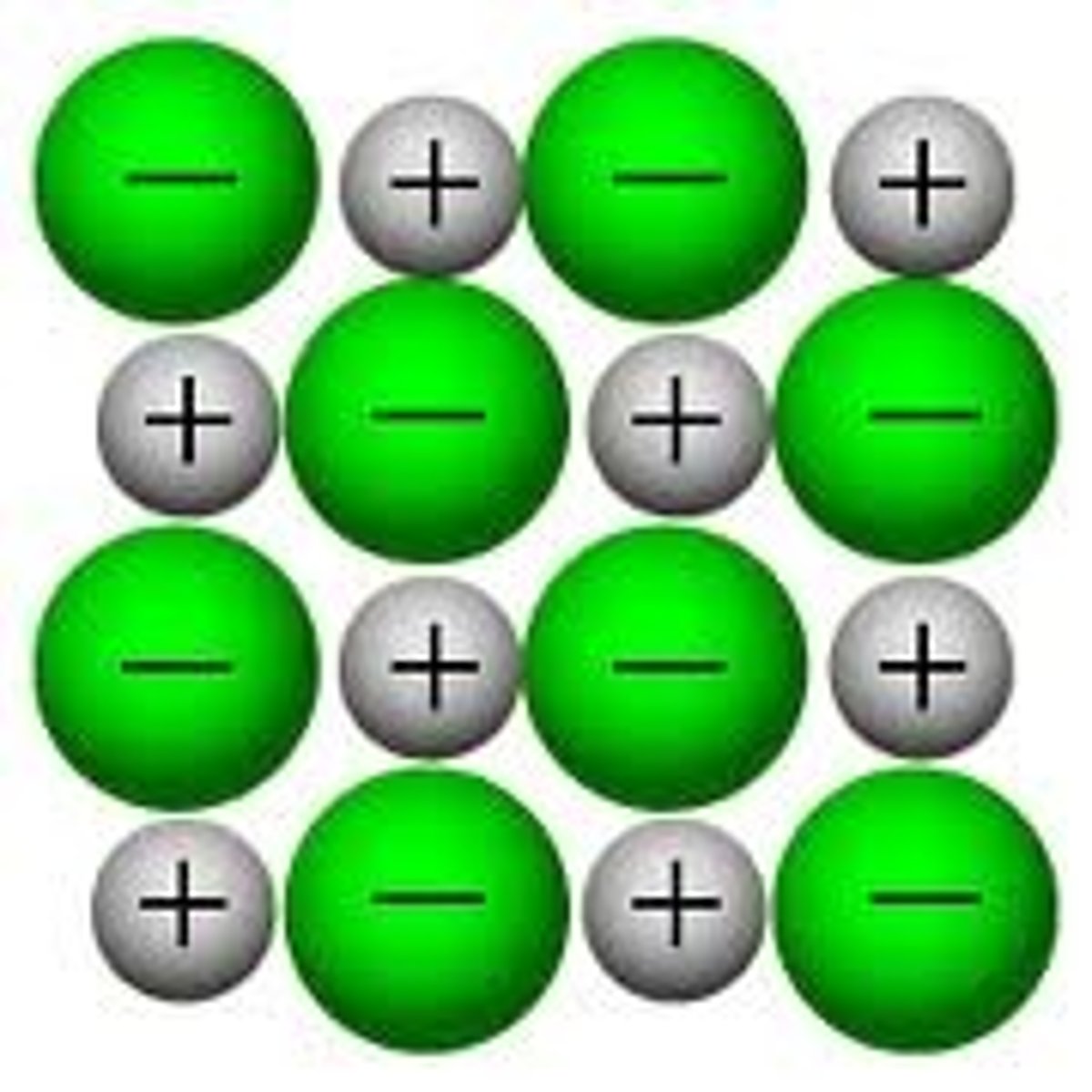
state the properties of ionic compounds
>high melting and boiling points- strong electrostatic forces of attraction between ions. especially in ionic compounds with greater charge due to strong attractions
>dissolve easily in water (polar solvents)- ions separate and are free to move in the solution. it is harder for water to break down ionic compounds with greater charge- forces may be too strong
>can carry an electrical current when molten or dissolved as they have mobile ions that can carry charge
cannot conduct as solids as there are no mobile ions.
Define covalent bonding
The strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
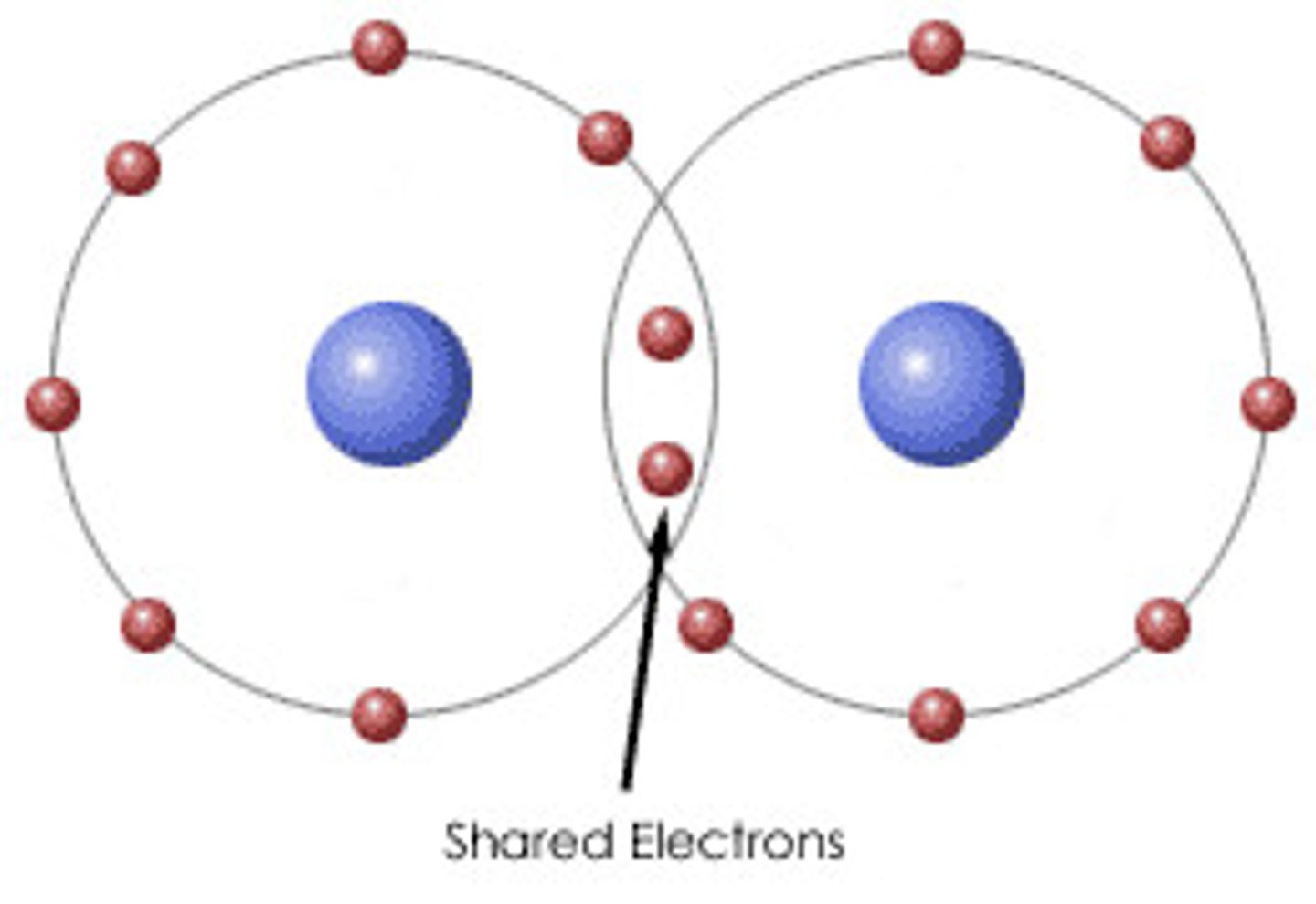
which compounds, ions or elements experience covalent bonding?
non metallic elements eg-O2
compounds of non metallic elements eg-H2O
polyatomic ions eg- NH4+
"a covalent bond is localised" what does this mean?
attraction is solely between the shared pair of electrons and the nuclei of the bonded atoms
what are the types of covalent bonds?
single (attraction between 1 shared pair and nuclei) double (attraction is between 2 chaired pairs and nuclei), and triple covalent bonds (attraction is between 3 shared pair and nuclei)
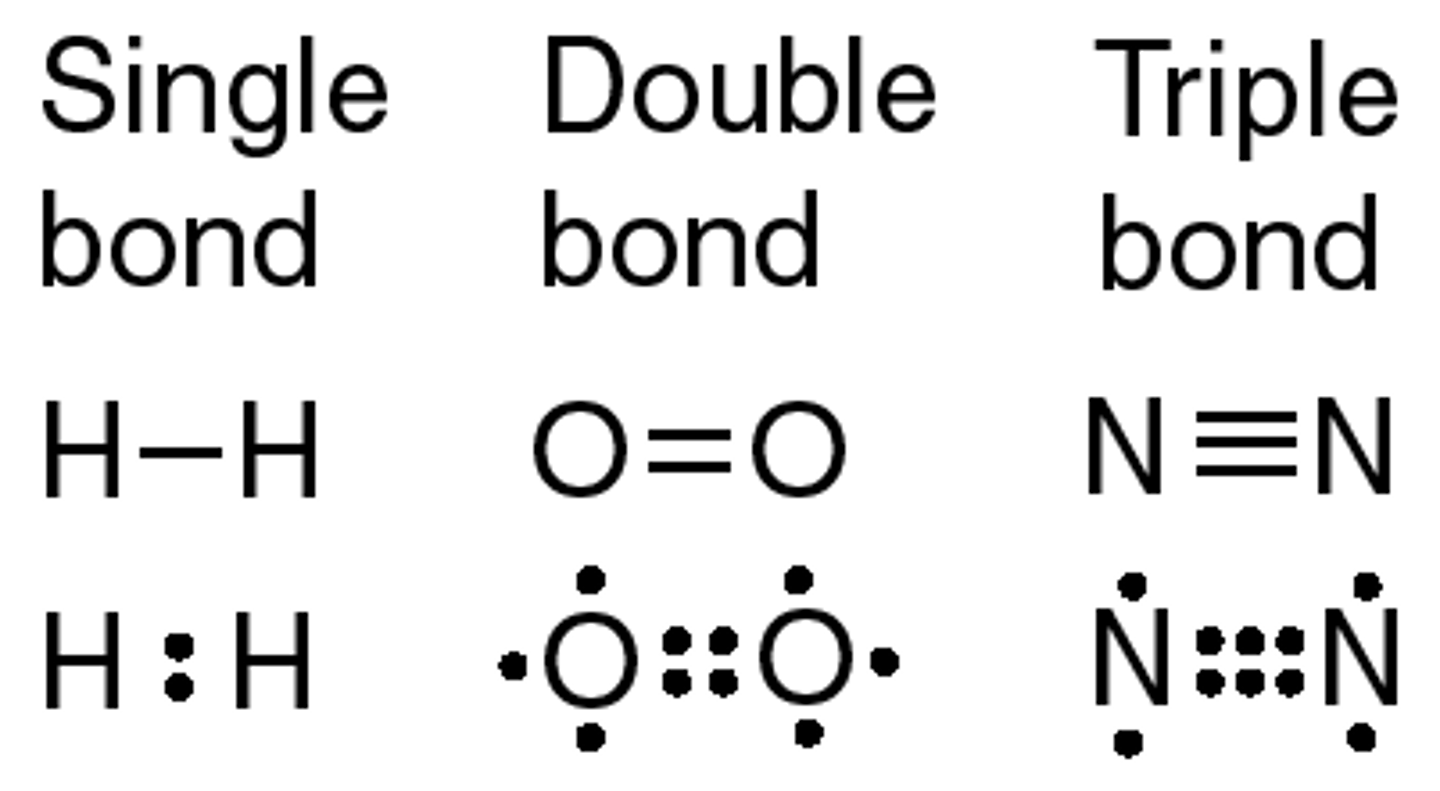
What is a dative covalent bond?
A covalent bond in which a shared pair of electrons is supplied by one of the bonding atoms only.
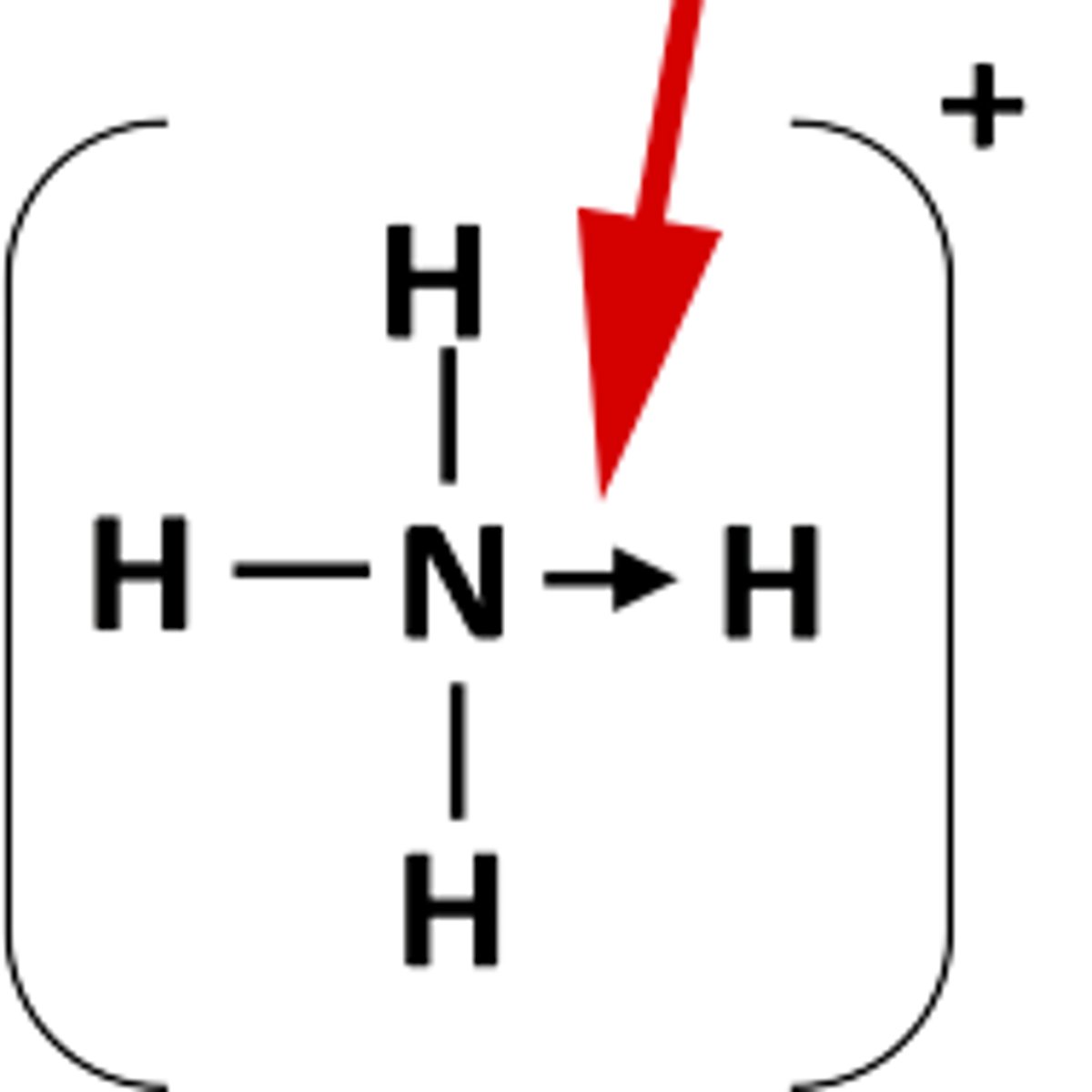
give some examples of dative covalent bonds
boron trifluoride and ammonia
define the term "acid"
a proton donor
What is a strong acid?
An acid which completely dissociates in water
What is a weak acid?
An acid which partially dissociates in water
Define an alkali
releases OH- ions in aqueous solutions
Define a base
a substance that readily accepts H+ ions
give the formula of common acids
hydrochloric acid- HCL
sulphuric acid- H2SO4
nitric acid- HNO3
ethanoic acid- CH3COOH
give the formula of common alkalis
sodium hydroxide- NaOH
potassium hydroxide- KOH
ammonia- NH3
Name some bases
metal oxides eg: MgO
metal carbonate eg: CaCO3
alkalis eg: NH3
metal hydroxides eg: Ca(OH)2
state the equation for the neutralisation of an acid by a metal oxide
acid+metal oxide=salt+water
state the equation for the neutralisation of an acid by an alkali
acid+alkali=salt+water
state the equations for the reaction of ammonia with acids
acid+ammonia=ammonium salt (eg: ammonium chloride)
What is titration?
the process of adding a known amount of solution of known concentration to determine the concentration of another solution
what are concordant titres?
Titres within 0.1cm³ of each other
what is a standard solution
a solution of known concentration
what is more accurate then using a beaker
a volumetric flask

How to prepare a standard solution?
1) weigh solid
2) dissolve solid in beaker using less water than needed to fill volumetric flask to the mark
3) transfer to volumetric flask. The last traces of solution are rinsed into the flask using distilled water
4) fill to graduation mark by adding distilled until bottom of meniscus lines up
5) invert volumetric flask several times to mix
acid-base titration steps
1) add a measured volume of one solution to a conical flask using a pipette
2) add the other solution to a burette and record the initial burette reading in cm3
3) add a few drops of indicator to the solution in the conical flask
4) run the solution in burette into the solution in conical flask, swirling to mix
5) to mark the end point, the indicator changes colour, towards the end add the solution drowpwise. this is used to tell you the volume of one solution that reacts with the volume of the second
6) record the final burette reading (the total volume added from the burette = the titre) (initial - final)
7) repeat to obtain more accurate results
titration calculations
[need to know]
- both the concentration and the reacting volume of ONE of the solutions
- only the reacting volume of the other
STEP 1 = work out the amount, in mol, of the solute in the solution of which you know both conc and volume
STEP 2 = use the equation to work out amount in mol kf other solution
STEP 3 = work out the unknown info about the other solute in the other solution
TIP for longer answer question
when asked to prep 250 cm3 of a solution, add 25 into a pipet then just times answer by 10 and scale up (or down if question is given in 250cm3)
identification of a carbonate (X)
first heat the water of crystallisation till mass is constant
1) prep unknown solution of X2CO3 in volumetric flask
2) using a pipette, measure 25.00cm3 of prepared solution into conical flask
3) using burger, titrate against 0.100 mol dm-3 HCL
-->

final part of analysis of identification of a carbonate ^^ (finding x)

burette

pipette

What is a redox reaction?
a reaction where oxidation and reduction have both occurred
what is reduction?
gain of electrons
What is oxidation?
loss of electrons
What is a reducing agent?
element that is oxidized
What is the oxidizing agent?
element that is reduced
what are the rules of assigning oxidation numbers?
1. The atoms in pure elements have an oxidation number of zero. i.e. Na, O₂, P₄ and S₈
2. Fluorine has an oxidation number of −1 in all of its compounds because it is the most electronegative element.
3. Oxygen has an oxidation number of −2 in almost all compounds. Exceptions include when it is in peroxides, such as H₂O₂, in which its oxidation number is −1, and when it reacts with fluorine, OF₂, in which its oxidation number is +2.
4. Hydrogen has an oxidation number of +1 in all compounds. it has an oxidation number of −1 in compounds with metals. i.e metal hydrides
5. The sum of the oxidation numbers of all atoms in a neutral compound has too equal to zero.
6. The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion, eg: in OH- they have to add to -1.
what is electron repulsion theory?
-Electron pairs repel each other meaning they
position themselves as far apart as possible.
-All bonding electron pairs repel each other
equally (and as far as they can)
-Lone pairs repel more than bonded
pairs.
State the bond angle, number of bonded pairs, and number of lone pairs in a trigonal planar molecule
3 bonded pairs
0 lone pairs
bond angle= 120*
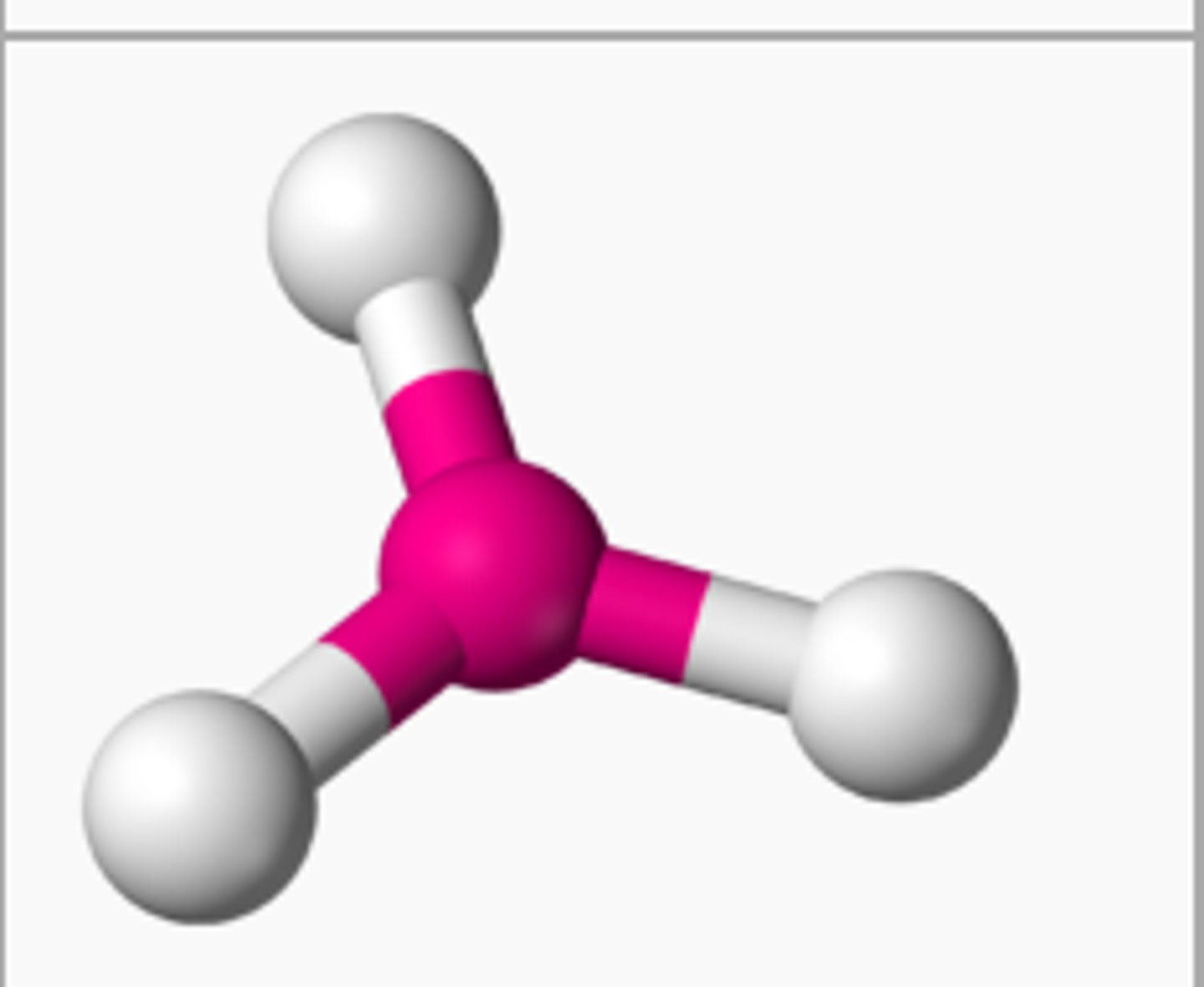
state the bond angle, number of bonding pairs, and number of lone pairs in a tetrahedral molecule
4 bonded pairs
0 lone pairs
bond angle= 109.5*
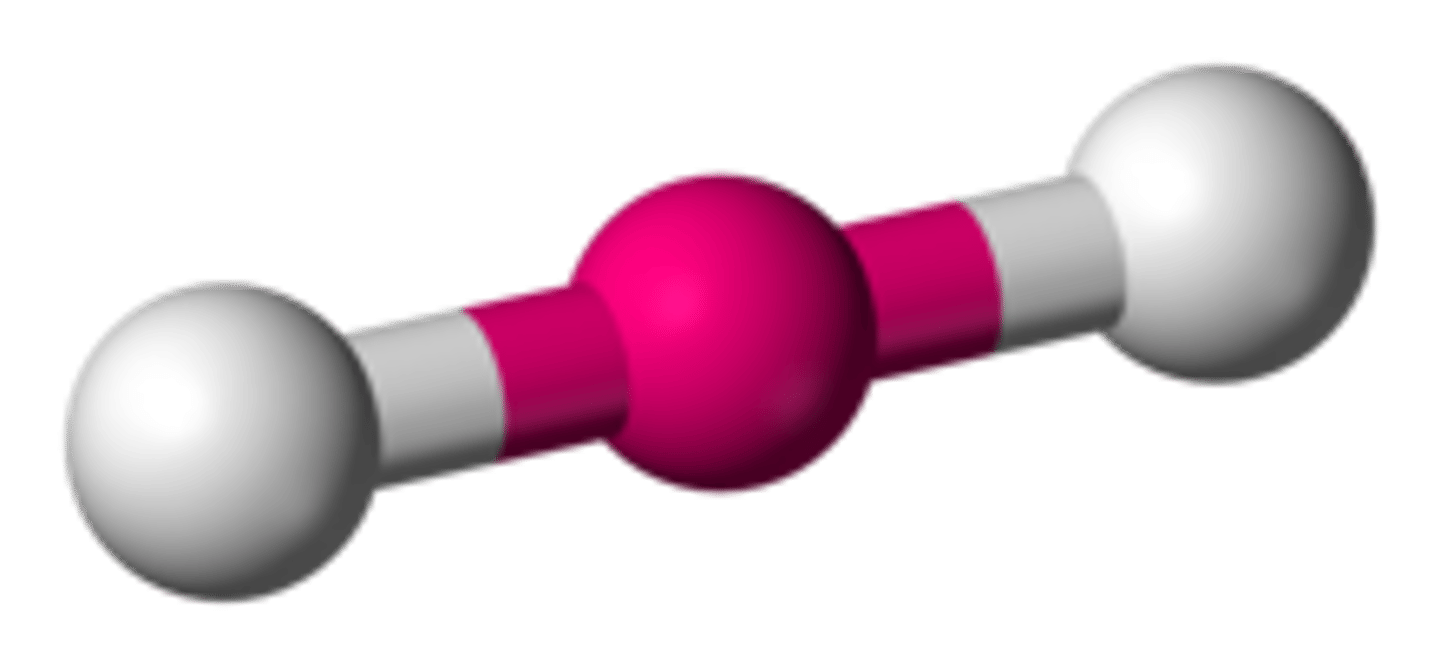
state the bond angle, number of bonding pairs, and number of lone pairs in a linear molecule
2 bonded pairs
0 lone pairs
bond angle= 180*
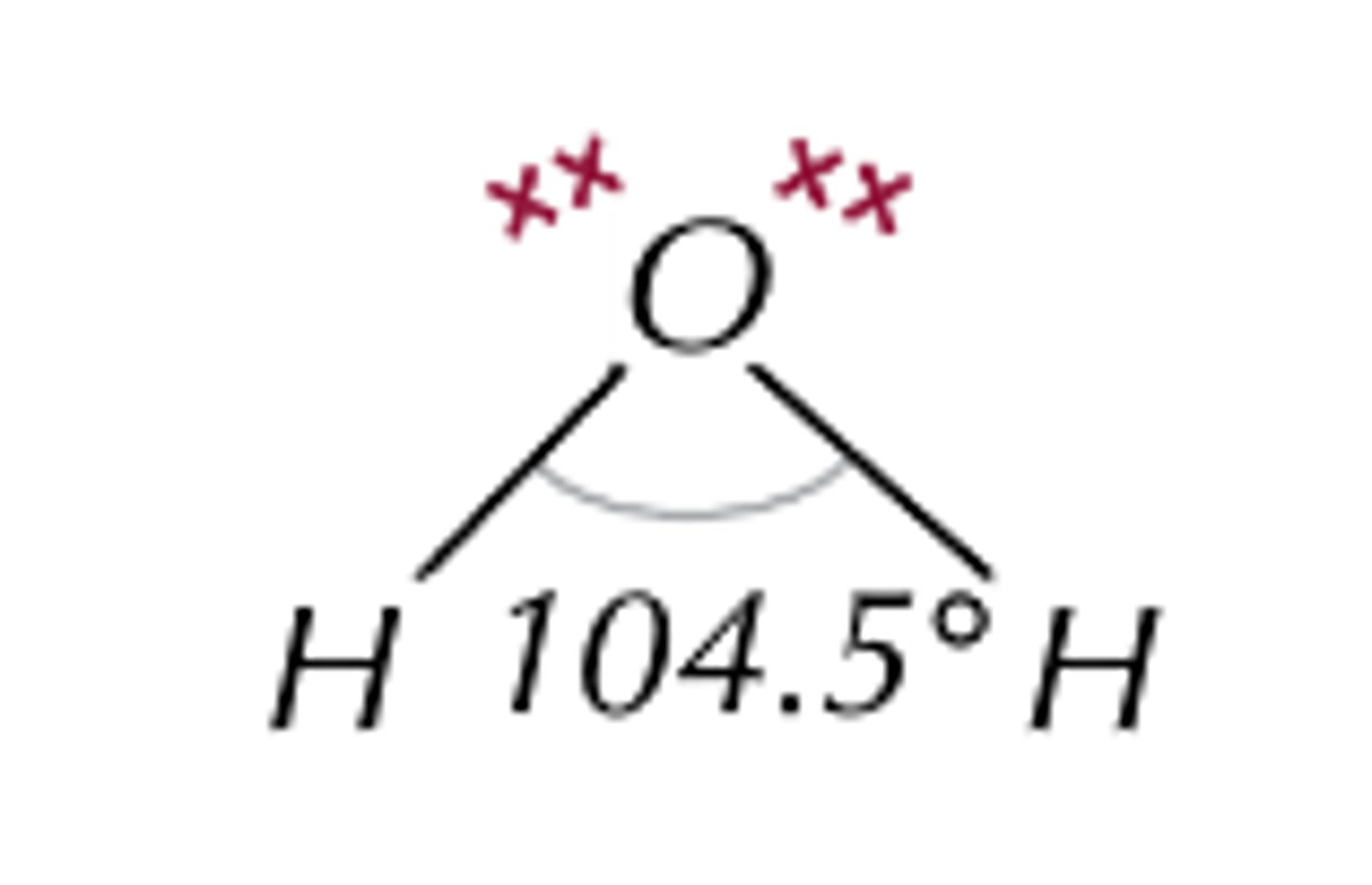
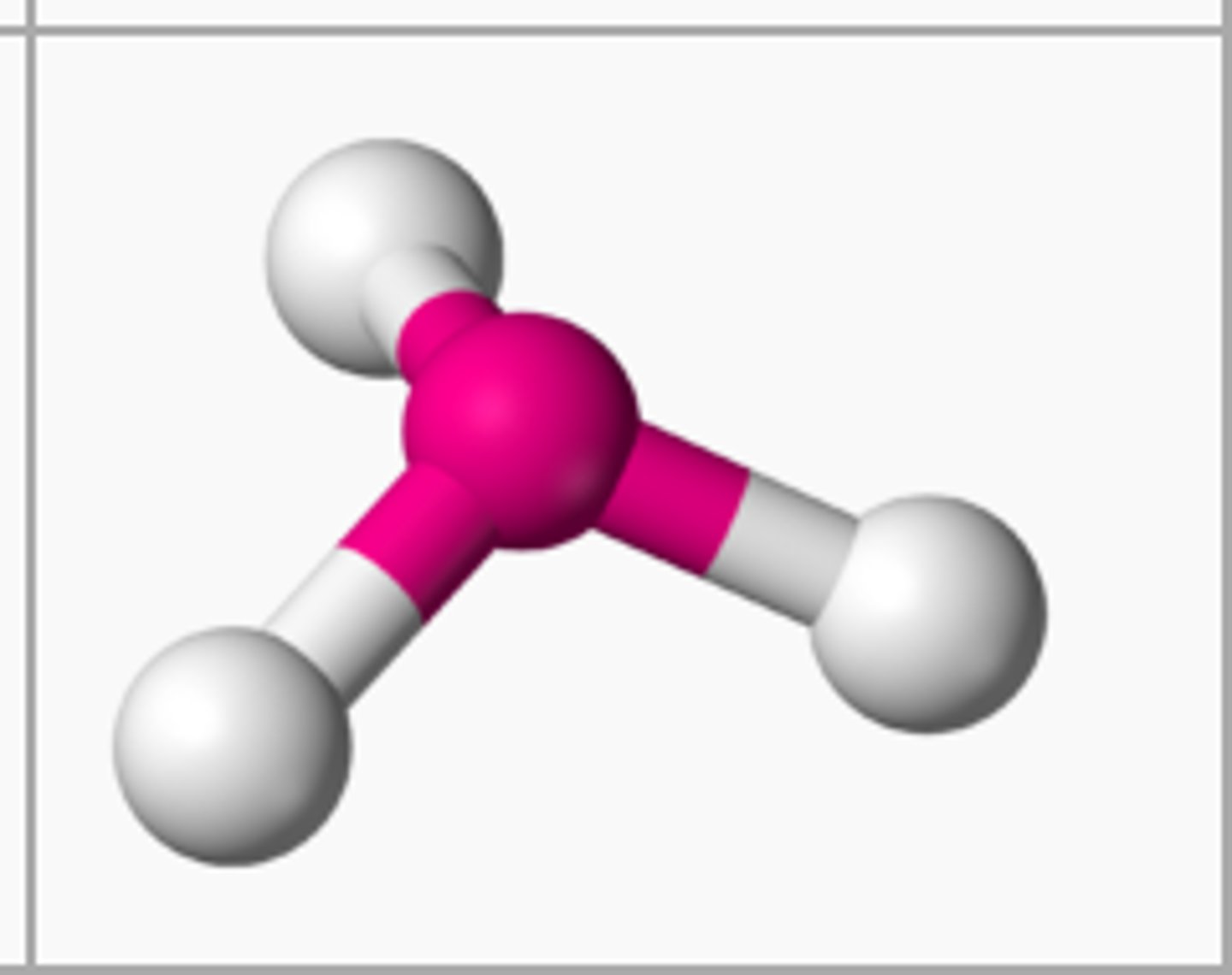
state the bond angle, number of bonding pairs, and number of lone pairs in a non linear (v shaped) molecule
2 bonded pairs
2 lone pairs
bond angle= 104.5*
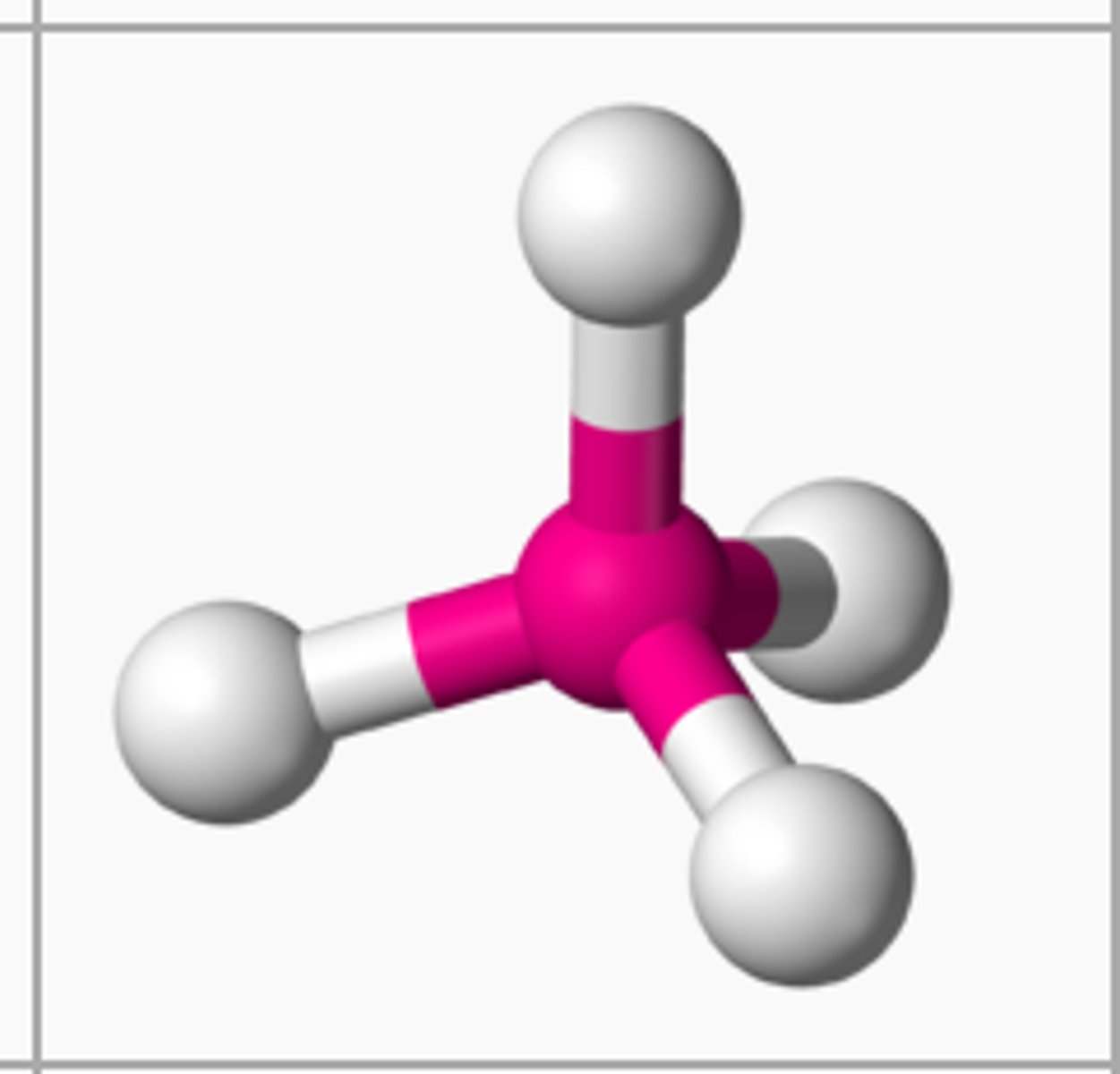
state the bond angle, number of bonding pairs, and number of lone pairs in a trigonal pyramidal molecule
3 bonded pairs
1 lone pair
bond angle= 107*
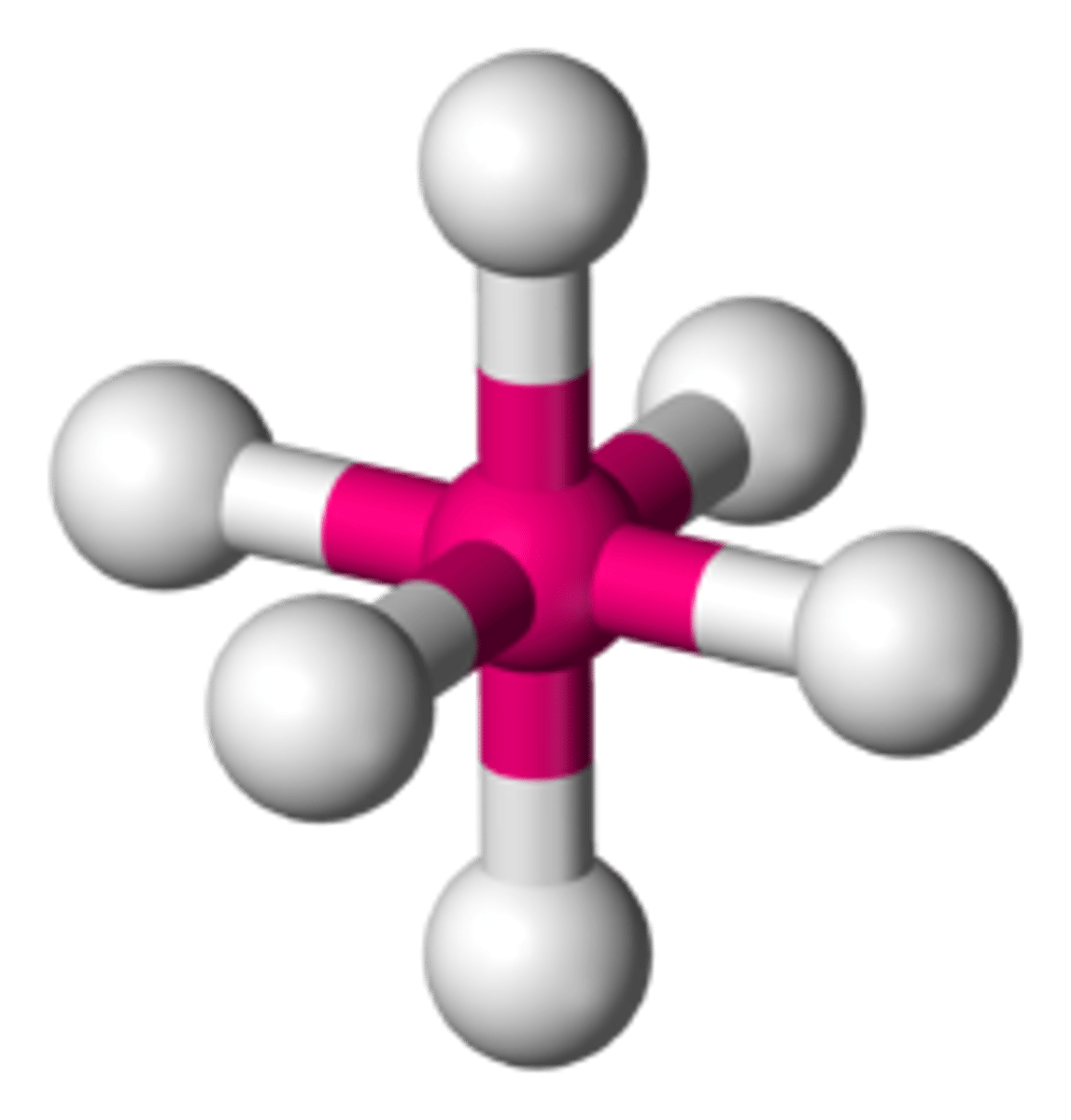
state the bond angle, number of bonding pairs, and number of lone pairs in a octahedral molecule
6 bonded pairs
0 lone pairs
bond angle= 90*
what does Paulings electronegativity scale state?
electronegativity depends on the size of the + charge on the nucleus. the more positive the charge on the nucleus the more attraction so more electronegativity
the more shells on an electron, the more shielding. as shielding increases, electronegativity decreases
what is a dipole?
the separation of charge
what are induced dipole dipole interactions? (London forces)
-the electrons in a molecule are constantly moving
-at any instant, an instantaneous (random) dipole will exist
-the instantaneous dipole induces a dipole on a neighbouring molecule, which further induces a dipole on neighbouring molecules and so on..
-they attract one another
what are the properties of London forces?
they are the weakest intermolecular force
they are easily broke
the more electrons, the stronger the London force
every atom experiences London forces
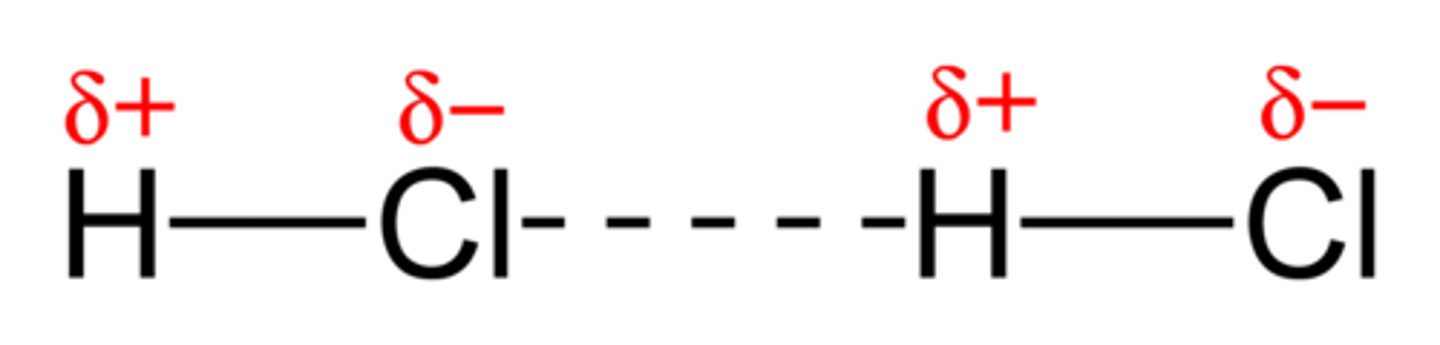
what are permanent dipole dipole interactions?
The attraction between permanent dipoles in different polar molecules.
the atoms involved have a dipole (difference in charge)
for example: HCl experiences permanent dipole dipole interactions
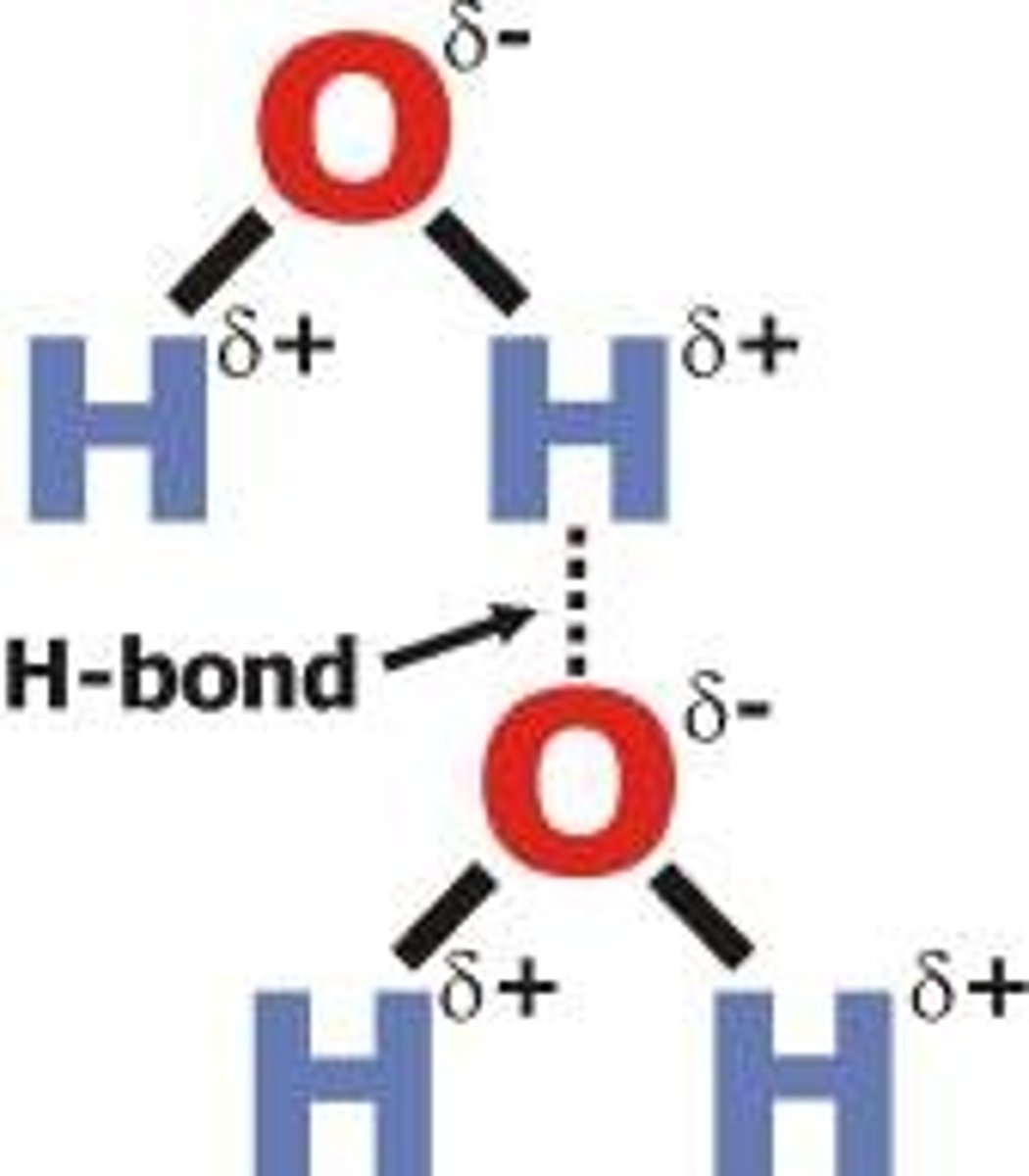
what is hydrogen bonding?
Hydrogen bonding is the strongest intermolecular force. it only happens when hydrogen is covalently bonded to fluorine, nitrogen or oxygen because they're very electronegative and can pull the bonding electrons away from the hydrogen.
Substances with hydrogen bonding have higher melting and boiling points because of the extra energy needed to break the hydrogen bonds.
state the properties of simple molecular substances
MELTING AND BOILING POINT
-low due to weak intermolecular forces (covalent bonds)
ELECTRICAL CONDUCTIVITY
-do not conduct in any state as they have no free mobile ions to carry charge
ARE INSOLUBLE
state the properties of giant covalent molecules (graphite, diamond, graphene and silicon)
MELTING AND BOILING POINT
-high due to very strong covalent bonds
ELECTRICAL CONDUCTIVITY
-diamond and silicone don't conduct in any state as they have no mobile ions
- graphene and graphite do as solid and molten as they have delocalised electrons.
ARE INSOLUBLE
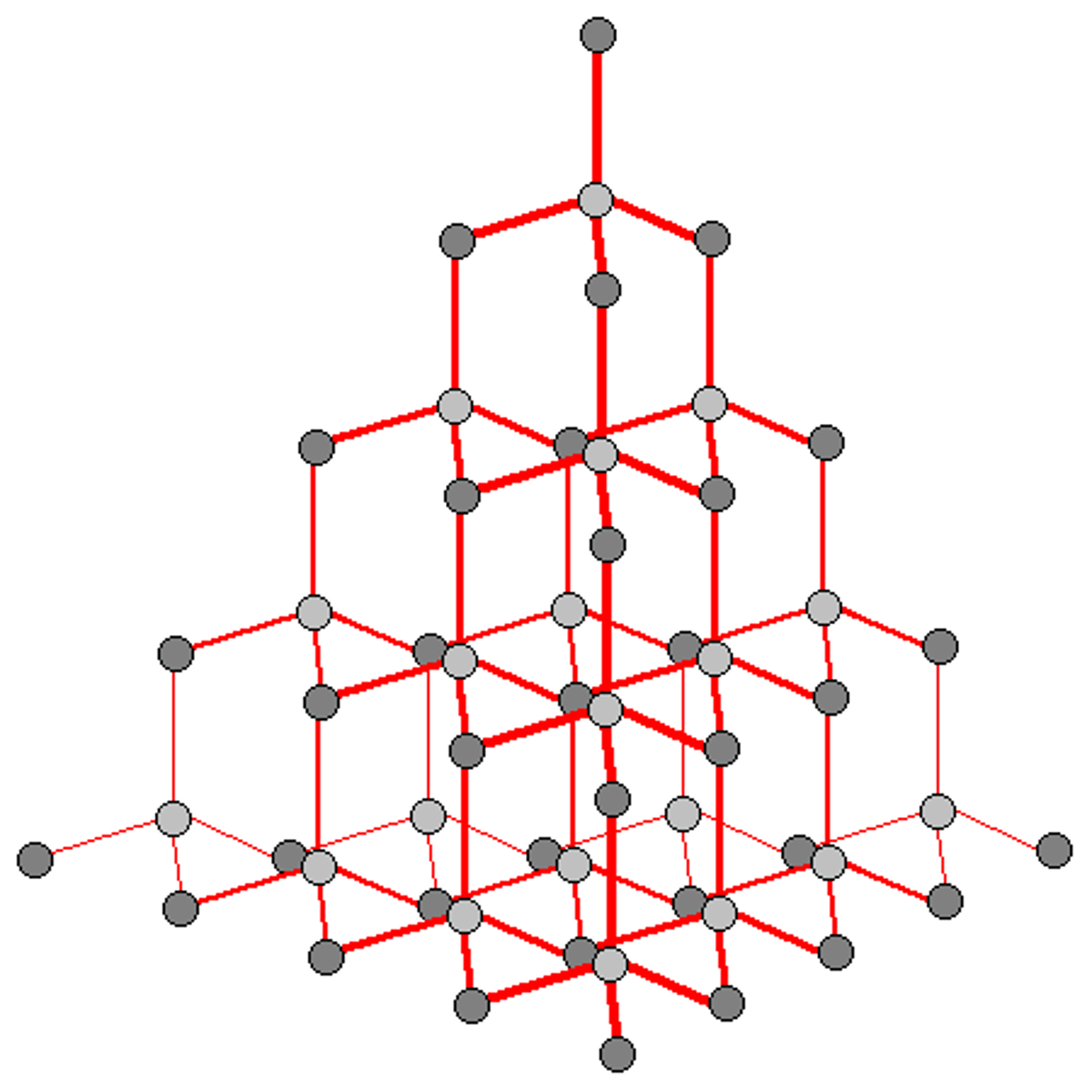
state the properties of giant ionic molecules
MELTING AND BOILING POINT
-high due to strong electrostatic forces of attraction
ELECTRICAL CONDUCTIVITY
-conduct only in molten or dissolved state due to free mobile ions
- do not conduct as solids
ARE SOLUBLE IN WATER