Unit 1- Chemistry [A2]- Production and uses of substances in relation to properties
1/172
Earn XP
Description and Tags
This is taken from the applied science textbook
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
173 Terms
What is atomic number?
the number of protons in an atom
What are periods?
horizonal rows
What are groups?
vertical columns
What increases as you move from left to right across the period?
atomic number
What groups are part of the s block?
groups 1 and 2
What groups are part of the p block?
groups 3 to 7 and group 0/8
What is part of the d block?
transition metals
Explain why calcium is an s block element
the last electron of calcium enters the s orbital
What is the only way to measure the radius of an atom?
to measure the distance between the nuclei of two touching atoms and divide by 2
What decreases across the period from left to right?
atomic radius
What leads to a decrease in atomic radius?
the extra protons increase nuclear charge, the increased nuclear charge attracts the extra electrons and pulls them closer to the nucleus
The atomic radii increases as…
you go down a group
Why is it that as you down a group, the atomic radii increases?
extra electrons are added to additional shells and the number of inner shells increases so the nuclear charge is shielded more
What is the trend for transition metals?
the atomic radii get slightly smaller as you go across the start of the transition metals but the radii stay similar.
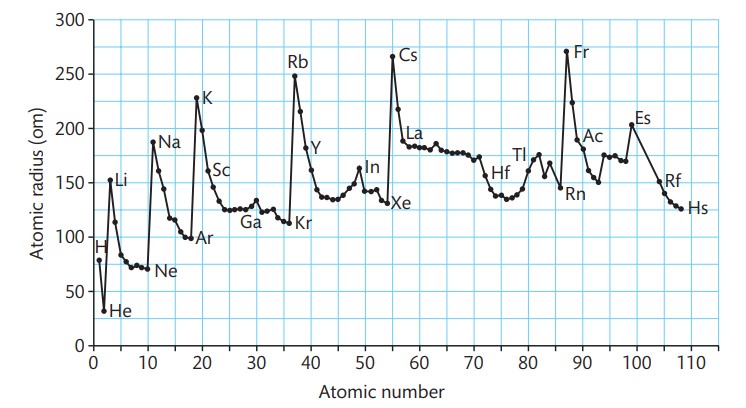
Why is the trend of transition metals like this?
This is because the additional nuclear charge is balanced by the extra shielding by the 3d electrons of the outer 4s sub-shell
The ionic radius down a group…
increases because extra electrons are added to extra shells as you go down, giving a larger size
What are cations?
ions with a positive charge
What has a smaller radius compared to a corresponding atom?
cations
All cations have the same electronic structure…
as you go across a periods
What are cations?
Cations are isoelectronic so the nuclear charge increases
What is the meaning of isoelectronic?
having the same number of electrons
What is the meaning of anion?
ions with a negative charge
anion have a large radius than corresponding atoms because…
there is more repulsion between extra electrons
As you go across the period…
anions are all isoelectronic
What also decreases as you go across the period?
ionic radius of the anion
What is electronegativity?
a measure of the tendency of an atom to attract a bonding pair of electrons
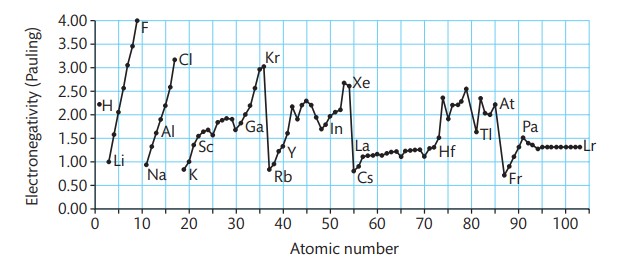
How does electronegativity increase or decrease?
increases as you go across a period and decreases as you go down a group
What does electronegativity depend on?
the number of protons in the nucleus, the distance from the nucleus of the bonding pair of electrons and how much shielding there is from inner electrons
What happens as you go down a group?
there is more shielding from inner electrons and the bonding pair of electrons are further from the nucleus. this adds up to less pull on the bonding pair of positive charge of the nucleus and so electronegativity decreases
Although trends in the periodic table are usually identified across periods or down groups, it can be…
diagonal to each other
What is the meaning of the first ionisation energy?
the energy needed for one mole of electron to be removed from one mole of gaseous atom
What is periodicity?
the repeating pattern seen by the elements in the periodic table
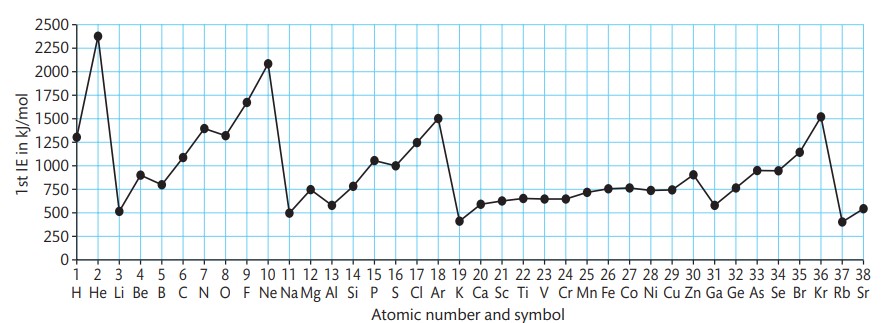
Describe the pattern of electron removal in periods 2 and 3.
Electrons removed from the third energy level are arranged in different sub-levels, with the first electrons in period 2 being removed from the 2s sub-level.
How does the ionization energy of beryllium compare to that of lithium?
Beryllium has a higher ionization energy than lithium because it has one more proton in its nucleus.
Define the reason for the decrease in ionization energy for boron compared to beryllium.
The decrease occurs because the electron removed from boron is taken from the 2p sub-level, which is at a higher energy level than the 2s sub-level, making it easier to remove.
Explain the shielding effect in relation to ionization energy.
The 2s sub-shell shields the 2p sub-shell, making it easier to remove an electron from the 2p sub-level.
How do the ionization energies of carbon and nitrogen compare?
Carbon and nitrogen show an expected increase in ionization energy because the electrons removed from each element are in the same 2p sub-level and are unpaired.
What is the significance of the second dip in ionization energy at oxygen?
The second dip occurs because the electron removed from oxygen is in the 2p sub-level and is paired with another electron, making it harder to remove.
Describe the arrangement of electrons in the 2p sub-level for carbon and nitrogen.
In carbon and nitrogen, the electrons removed are unpaired and occupy their orbitals on their own.
Describe the effect of electrostatic repulsion on ionisation energy.
Electrostatic repulsion between two electrons in an orbital makes it easier to remove one of the electrons, leading to a decrease in ionisation energy.
How does the first ionisation energy change for fluorine and neon?
The first ionisation energy increases for fluorine and neon due to their increasing positive charge.
Define the trend of first ionisation energy in period 3.
In period 3, the first ionisation energy increases as electrons are removed from the 3s and 3p sub-levels.
Explain the trend of first ionisation energy in period 4 for groups 1 to 7 and 0.
In period 4, a similar trend is observed where the first ionisation energy increases across groups 1 to 7 and 0.
What is unique about the outer electron in transition elements?
In transition elements, the outer electron always comes from a 4s sub-shell, which has a higher energy than the 4d sub-shell.
How does the number of protons and 3d electrons affect ionisation energy in transition elements?
As you go across the transition elements, the number of protons increases, but the 3d electrons provide shielding, which cancels out the effect of the extra protons, resulting in only a slight increase in ionisation energy.
Describe the general trend of first ionisation energy as you go down a group.
The first ionisation energy generally falls as you go down a group.
Describe the relationship between the number of protons and ionisation energy in sodium compared to lithium.
Sodium has 8 more protons than lithium, which might suggest a higher first ionisation energy due to increased positive charge. However, the outer electron in sodium is further from the nucleus and experiences more shielding, resulting in a lower first ionisation energy.
How does electron shielding affect ionisation energy in sodium and lithium?
In lithium, the outer electron is shielded by 2 negative electrons and attracted to 3 positive protons, resulting in an overall attractive charge of +1. In sodium, the outer electron is shielded by 10 negative electrons and attracted to 11 positive protons, also resulting in an overall attractive charge of +1. However, the greater distance from the nucleus in sodium reduces the effective nuclear charge felt by the outer electron, lowering its ionisation energy.
Define ionisation energy and its trend in group elements.
Ionisation energy is the energy required to remove an electron from an atom. In group elements, such as groups 1, 2, and 7, the ionisation energy generally decreases as you move down the group due to increased distance from the nucleus and increased electron shielding.
Explain why sodium has a lower first ionisation energy than lithium despite having more protons.
Sodium has a lower first ionisation energy than lithium because its outer electron is further from the nucleus and experiences more shielding from inner electrons, which reduces the effective nuclear charge acting on it.
How does the distance of the outer electron from the nucleus influence ionisation energy?
The greater the distance of the outer electron from the nucleus, the lower the ionisation energy, as the attractive force from the nucleus decreases and the effect of electron shielding increases.
Describe the overall attractive charge experienced by the outer electron in lithium.
In lithium, the outer electron is attracted to 3 positive protons and is shielded by 2 negative electrons, resulting in an overall attractive charge of +1.
Describe the overall attractive charge experienced by the outer electron in sodium.
In sodium, the outer electron is attracted to 11 positive protons and is shielded by 10 negative electrons, resulting in an overall attractive charge of +1.
What trend is observed in ionisation energy as you move down group elements?
As you move down group elements, the first ionisation energy generally decreases due to increased distance from the nucleus and increased electron shielding.
What is the definition of electron affinity?
the change in energy when one mole of electrons to form a mole of negative ion
Describe the significance of the negative sign in energy release.
The negative sign indicates that energy is released during a process.
How does the amount of energy released change as you go down group 7?
The amount of energy released generally decreases as you go down group 7, with fluorine being an exception.
Define electron affinity.
Electron affinity indicates the strength of attraction between the nucleus of an atom and an incoming electron.
What factors affect electron affinity?
Factors affecting electron affinity include the number of protons (nuclear charge), distance from the nucleus, and shielding.
Explain the trend of nuclear charge as you move down a group.
As you go down a group, the nuclear charge increases due to the addition of protons.
How does shielding affect electron affinity as you go down a group?
Extra shielding from electrons in further shells reduces the attraction of the nucleus, weakening electron affinity.
What happens to the attraction between the nucleus and the outer shell as you go down a group?
The attraction becomes weaker as the outer shell is further from the positive pull of the nucleus.
Describe the unique behavior of fluorine regarding electron affinity.
Fluorine does not follow the trend of decreasing electron affinity because it is a very small atom, and the new electron experiences repulsion from already filled electron regions.
How does the electron affinity of oxygen compare to that of sulfur?
Oxygen has a lower electron affinity than sulfur, similar to how fluorine has a lower electron affinity than chlorine.
What is the general trend of electron affinities in group 6 as you move down the group?
Electron affinities in group 6 generally decrease as you go down the group.
Compare the electron affinities of group 6 and group 7 elements in the same period.
Group 6 elements have lower electron affinities than group 7 elements in the same period due to having one less proton but the same amount of shielding.
What is the relationship between elements in a period regarding electron shells?
Elements in a period have the same number of electron shells.
Describe the second electron affinity for Group 6 elements.
The second electron affinity for Group 6 elements occurs when a negative ion gains a second electron, forming a charge of –2. This process involves a positive change in energy because the two negative charges repel each other, requiring energy to add the electron.
How does the second electron affinity for oxygen compare to its first?
The first electron affinity for oxygen is –142 kJ mol–1, while the second electron affinity is +844 kJ mol–1, indicating that a significant amount of energy is required to overcome the repulsion between the two negative charges.
Define electronegativity and its role in bonding.
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. It helps predict the type of bonding in a compound, ranging from ionic to covalent.
Explain the bonding in a hydrogen molecule.
In a hydrogen molecule, both hydrogen atoms have the same electronegativity, resulting in a non-polar covalent bond.
What type of bond is formed when hydrogen bonds with fluorine?
When hydrogen bonds with fluorine, a polar covalent bond is formed due to fluorine's high electronegativity, which attracts the bonding pair of electrons more strongly.
How did Linus Pauling contribute to the understanding of electronegativity?
Linus Pauling developed a scale that provides relative values for the electronegativity of elements, allowing predictions about the ionic or covalent nature of bonds.
Describe the spectrum of bonding types in compounds.
Bonding in compounds exists on a spectrum from ionic to covalent, with most compounds exhibiting characteristics of both types rather than being wholly ionic or covalent.
What happens to the energy change when a second electron is added to a negative ion?
The energy change is positive because energy is required to overcome the repulsion between the two negative charges when adding a second electron.
Describe the relationship between electronegativity difference and bond polarity.
A low difference in electronegativities results in a less polar covalent bond, while a high difference increases polarity. If the difference is very large, the bond becomes ionic.
Define ionic bond polarization.
Ionic bond polarization refers to the distortion of the electron cloud of an anion due to the presence of a cation, influenced by the charge and size of the ions.
How does the size and charge of ions affect ionic bond characteristics?
A small, highly charged cation draws electrons towards it, while a large, highly charged anion has a distorted electron cloud, leading to some covalent characteristics in the ionic bond.
Explain the trend of melting and boiling points in Group 1 elements.
In Group 1, as you go down the group, the melting and boiling points decrease, indicating that the forces of attraction between the atoms weaken.
What happens to melting and boiling points in Group 7 elements as you move down the group?
In Group 7, the melting and boiling points increase as you go down the group, indicating that the forces of attraction between the atoms strengthen.
How does energy relate to the melting and boiling processes of elements?
When an element melts, energy is used to overcome some attractive forces between atoms or molecules. When it boils, most of the remaining attractive forces are broken.
Define the factors that influence the strength of melting and boiling points in elements.
The strength of melting and boiling points depends on the strength of the forces between the atoms in an element.
Describe the characteristics of a small cation and a large anion in ionic bonds.
A small cation that is highly charged tends to attract electrons, while a large anion that is highly charged has an easily distorted electron cloud, affecting the bond's characteristics.
Describe the trend of melting and boiling points across periods 2 and 3.
Melting and boiling points peak in the middle of periods 2 and 3, with the lowest values found in group 0.
How does metallic bonding affect melting and boiling points in groups 1 to 3?
As you go across groups 1 to 3, metals have increasing nuclear charge and delocalised electrons, leading to stronger metallic bonding and higher melting and boiling points.
Define the bonding structure of carbon and its effect on melting and boiling points.
Carbon has a giant covalent bonding structure forming a giant lattice, with each atom bonding to 4 other carbon atoms, resulting in very high melting and boiling points due to strong covalent bonds.
Explain the melting and boiling points of non-metals in groups 5 to 7.
Non-metals in groups 5 to 7 have small separate molecules, resulting in low melting points due to weak van der Waals forces that need to be overcome.
How does sulfur's melting and boiling points compare to other non-metals?
Sulfur has a higher melting and boiling point than other non-metals because it has a larger molecular size (S8) compared to others like P4 and Cl2.
What role do van der Waals forces play in the melting and boiling points of elements?
The strength of van der Waals forces increases with the size of the molecule; larger molecules have stronger forces, leading to higher melting and boiling points.
Describe the molecular forms of phosphorus, sulfur, chlorine, and argon.
Phosphorus exists as P4, sulfur as S8, chlorine as Cl2 molecules, and argon as Ar atoms.
How do the melting and boiling points of sulfur compare to those of phosphorus and chlorine?
Sulfur has higher melting and boiling points than phosphorus and chlorine due to its larger molecular size and stronger van der Waals forces.
What does metallic bonding allow?
allows for electrical conductivity through a solid or liquid metal
What is an example of a conductor of electricity?
copper
Are metals good thermal conductors?
yes
What does the structure of metals explain?
that they can be malleable or ductile
What is the meaning of malleable?
can be hammered into shape without breaking
What is the meaning of ductile?
can be hammered thin or stretched into wires without breaking
Why is it that the reactions between oxygen and metals are very important?
how they react can influence how a metal is used
What is the meaning of alkaline solution?
a solution with a pH above 7
What is the meaning of oxidation?
loss of electrons from an atom/ion