Lesson 1
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
How does nucleophilicity change?
More nucleophilic when more negatively charged
Increases as size increases (as you go down)
Increases as electronegativity decreases (as you go left)
What are nucleophiles?
Lewis bases (donate electrons to form covalent bonds)
Donate electrons
Ex: OH-, halogens, H2O, ROH, RNH2, etc.
What are electrophiles?
Lewis acids (gain electrons through covalent bonds)
Receive electrons
They are electron deficient
Ex: carbocations, carbonyl compounds, alkyl halides; O and halogens create partial charges
What are leaving groups?
When the electrophile receives electrons, it will kick off something else it is attached to (leaving group)
What makes a good leaving group? Bad?
Good: stable in solution (resonance, weak base (increases with size), neutral)
Bad: unstable in solution (strong bases. hydrogens, most carbons)
How do you make a bad leaving group a good one?
Protonate it (aka acid catalysis)
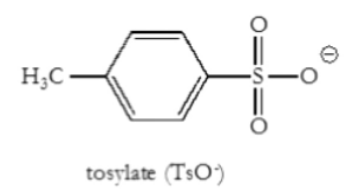
What is TsO-?
Tosylate
Good leaving group
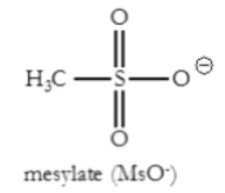
What is MsO-?
Mesylate
Good leaving group
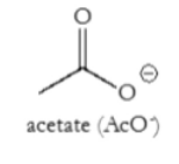
What is AcO-?
Acetate
Good leaving group
What are constitutional isomers?
Same formula, different connections
Different chemical and physical properties
What are conformational isomers?
Same formula, same connections, different rotation around a sigma bond
Same physical and chemical properties
Cannot be isolated
Eclipsed or staggered
What are stereoisomers?
Same formula, same connectivity, different spatial arrangement
Ex: enantiomers, diastereomers, epimers
What are chiral molecules?
Do not have symmetry about the atom
No plane of symmetry
Not superimposable
How to determine R and S?
Locate priority
If lowest priority is back (dashed lines), then R is clockwise and S is counterclockwise
If lowest priority is not back switch directions of R and S
What are enantiomers?
Non-superimposable mirror images
Same physical characteristics
Have opposite configuration of all chiral centers
Same magnitude of rotation, but opposite sign
Separate them via resolution
What are diastereomers?
Non-superimposable non-mirror images
Different characteristics
Opposite configuration for at least one, but not all, chiral centers
Unrelated optical activities
Can be physically separated
What are epimers?
Diastereomers that differ at one single chiral carbon
All epimers are diastereomers, but not the inverse
What are anomers?
Molecules that can be cyclic (AKA sugars)
C1 is thus an anomeric center (two configurations)
What are meso compounds?
With an internal plant of symmetry in a molecule with > 1 chiral centers
Symmetrical chiral centers have opposite configurations
Optically inactive
What are geometric isomers?
Restricted rotation about a double bond or ring
Do not require chiral centers
All physical and chemical properties are different
Type of diastereomer
Cis vs trans
E vs Z
How to calculate degrees of unsaturation?
2n + 2 H’s expected if the hydrocarbon is saturated
[(2n + 2) - #H]/2 = DOU
Treat halogens as hydrogen, ignore oxygens, subtract the number of nitrogens from the number of Hs
What is the relationship between stability and reactivity?
Strong inverse
What does ring strain do?
Destabilizes the ring
Weakens C-C bonds
Increases reactivity
What is induction?
Electrons in a sigma bond will shift towards the more electronegative atom
Electronegative substituents withdraw electron density, while electropositive ones do the opposite (donate)
What are electron withdrawing groups?
Halogens, oxygen
What are electron donating groups?
Alkyl groups (CxHy)
What is the stability of carbocations from most to least?
Given R carbocations are attached to R groups at a carbon
Most: tertiary (three groups to push electrons towards the carbocation) > secondary > primary >
Least: methyl (no groups to push electrons towards the carbocation)
What is the stability of carbanions from most to least?
Most: methyl > primary > secondary > tertiary
Alkyl R groups donate electrons —> more negative things towards a negative charge —> unstable
What is resonance stabilization?
Stabilization due to delocalization of electrons
- or + charge or lone electron pair next to a pi bond causes it
What are rules of resonance?
More resonance structures = more stable
Delocalization is only though p-orbitals (cannot be sp hybridized)(must have a double/triple bond of some sort)
Lowest energy structures contribute the most to the hybrid
When is a resonance structure low in energy?
Octet rule satisfied (most important)
Lowest formal charge
Negative charges on more electronegative atoms
How is the strength of an acid determined?
Relative stability of conjugate base
Acids donate protons more easily when the conjugate base is stable (therefore are more acidic)
What makes a conjugate base stable?
Negative charge on a more electronegative atom
Resonance increases stability of anion
Induction: proximity of electronegative atom to an electron withdrawing group (halogens, O)
Also how many electron withdrawing groups are present
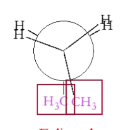
What is staggered conformation?
Substituents do not overlap in a plane
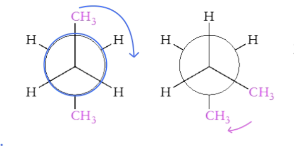
What is eclipsed conformation?
Substituents overlap in a plane
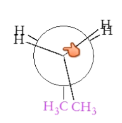
What is the anti conformation?
Two largest substituents 180 degrees from one another
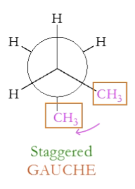
What is the gauche conformation?
Two largest substituents are next to one another, but not eclipsed
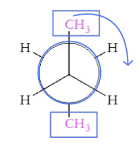
What is syn conformation?
Two largest substituents on top of each other and eclipsed
What are the conformational isomers (newman projections) ranked in stability? Energy?
Most stable: anti > gauche > syn
Most energy: syn > gauche > anti
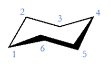
What is the chair cyclohexane?
See image
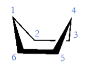
What is the boat cyclohexane?
See image
What is the axial position? Equatorial?
Axial: in boat/chair, when substituents point up or down
Equatorial: in boat/chair, when substituents point diagonal
What does a ring flip do?
See image
Axial → equatorial
Equatorial → axial
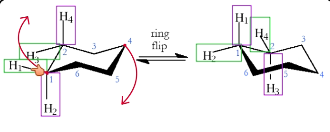
Is axial or equatorial more stable?
Equatorial
Axial has more steric strain
What ring conformation is most stable?
Boat, with most substituents in equatorial positions
What are characteristics of a chiral center?
AKA: stereocenter, stereogenic center, asymmetric center
sp³ hybridized
Tetrahedral geometry
Four different substituents
How do you know how many isomers a molecule has?
2^n
n = # of chiral centers
What does it mean to be optically active?
Chiral molecule that rotates plane-polarized light
Cannot be predicted based on structure
Must measure via polarimeter experimentally
What is dextrorotary? Levorotary?
Dextrorotary (d): + optical activity, clockwise rotation
Levorotary (l): - optical activity, counterclockwise rotation
How are substituents prioritized?
Atom with highest atomic number has highest priority
Heavier isotopes have higher priority
If two atoms are identical, move to the first point of difference → use above rules
Atoms with double bonds count as two of them
How do you assign R and S?
Lowest priority should face the back (dashed wedge)
Trace circle from 1-4 priority
Clockwise is R, counterclockwise is S
If you had to move things to make the lowest priority be back, switch R and S
How to do hand trick for R and S?
Put thumb in position of lowest priority
Curl hand to follow path of 1-3 priorities
If right hand curls correctly, it is R; if left hand works, it is S
How do you decide R or S for fischer projections?
Groups on the horizontal are towards you, groups on the vertical are away
Put rest of fingers towards 1
Curl fingers to 2
If right hand works = R; left hand = S
When do you use E vs Z?
When there is a double bond
E: trans
Z: cis