L22 mood disorders and antidepressants 2
1/95
Earn XP
Description and Tags
depression and bipolar 2
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
What are the heritability estimates for Bipolar Disorder and Major Depression?
Bipolar Disorder: ~93% heritability
Major Depression: ~37% heritability
✅ Suggests a stronger genetic contribution in bipolar disorder
Which neurotransmitters are implicated in mood disorders?
Dysfunction in monoamines:
Serotonin (5HT)
Noradrenaline
Dopamine
What are two biological processes linked to mood disorders beyond neurotransmitters?
Neuroendocrine dysfunction:
Disruption of the hypothalamic-pituitary-adrenal (HPA) axis
Neurogenesis:
Decreased rate of new neuron formation in the adult hippocampus
how might neuroinflammation contribute to mood disorders?
Chronic inflammatory conditions may increase cytokines
These can cross the blood-brain barrier and activate microglia, contributing to mood dysregulation
What are some key psychosocial/environmental risk factors for mood disorders?
Stressful life events (e.g. marriage breakdown, job loss)
Co-morbid conditions: chronic pain, substance abuse
Medications: corticosteroids, oral contraceptives, interferon
Which neurotransmitters (monoamines) are most implicated in mood disorders?
Serotonin (5-hydroxytryptamine or 5-HT)
Noradrenaline (NA)
Dopamine (DA)
These are collectively known as the monoamines.
What neurotransmitter levels are typically low in Major Depressive Disorder (MDD)?
Low levels of:
5-HT (serotonin)
Noradrenaline
Possibly dopamine
What neurotransmitter levels are typically found in mania?
High levels of:
Noradrenaline
Dopamine
Low levels of:
5-HT (serotonin)
Mania:High levels of noradrenaline and dopamine are implicated in manic episodes, which align with your notes that mania is characterized by high noradrenaline and dopamine, while serotonin is typically low.
Monoamine hypothesis in mania: While depression is linked to low monoamine levels, mania is thought to involve a dysregulation or excess of dopamine and noradrenaline.
Low serotonin in mania: Contrary to common ideas that serotonin might be high in mania, some studies suggest that low serotonin can contribute to the disinhibition and impulsivity seen in manic states.
The Link:
Monoamine hypothesis: The idea that mood disorders (like MDD and mania) stem from imbalances in neurotransmitters, specifically the monoamines (serotonin, dopamine, and noradrenaline), is a key framework for understanding mood disorders.
Depression tends to involve low serotonin, noradrenaline, and dopamine, while mania often involves high dopamine and noradrenaline and low serotonin.
So, the idea that mania involves low serotonin and high dopamine/noradrenaline fits the traditional view of how these neurotransmitters are thought to contribute to mood disorders. While serotonin’s exact role in mania can be complex, low serotonin is often seen as contributing to mood instability and impulsivity during manic episodes.
What is the monoamine hypothesis of depression, and what is it based on?
Suggests that depression is caused by low levels of monoamines
Based on the fact that many antidepressant drugs increase monoaminergic transmission
How does Reserpine support the monoamine hypothesis?
Inhibits VMAT, inhibits storage of 5-HT and noradrenaline
Without VMAT, neurotransmitters can't be properly stored inside vesicles.
When the neuron fires, there are fewer neurotransmitters available to be released because they haven’t been properly stored in the vesicles. As a result, serotonin and noradrenaline are depleted from both the vesicles and the synapse.
Associated with lowered mood
Supports the monoamine hypothesis
What happens when tryptophan (a serotonin precursor) is depleted in the brain?
Lowers brain 5-HT synthesis
Can induce relapse in previously recovered MDD patients
what is the monoamine hypothesis?
that depression is a functional deficit of 5ht and/or noradrenaline in the brain
5ht is responsible/linked to?
obsession
compulsion
memory
noradrenaline is linked to?
alertness
concentration
energy
attention
dopamine is linked to?
attention
reward
motivation
What is the role of the ventral hippocampus (HP) in mental functioning?
Supports cognitive function and memory
Linked to stress response and mood regulation
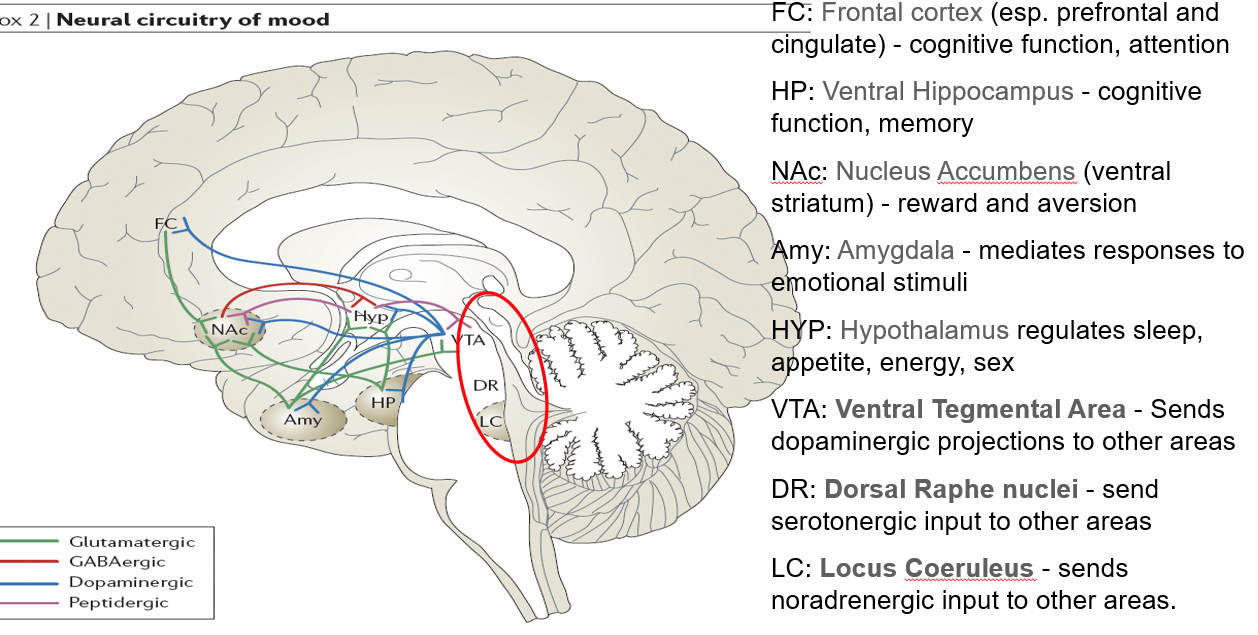
What function does the nucleus accumbens (NAc) serve in the brain?
Part of the ventral striatum
Regulates reward, motivation, and aversion
Involved in pleasure-seeking behaviors (relevant in mood and addiction)
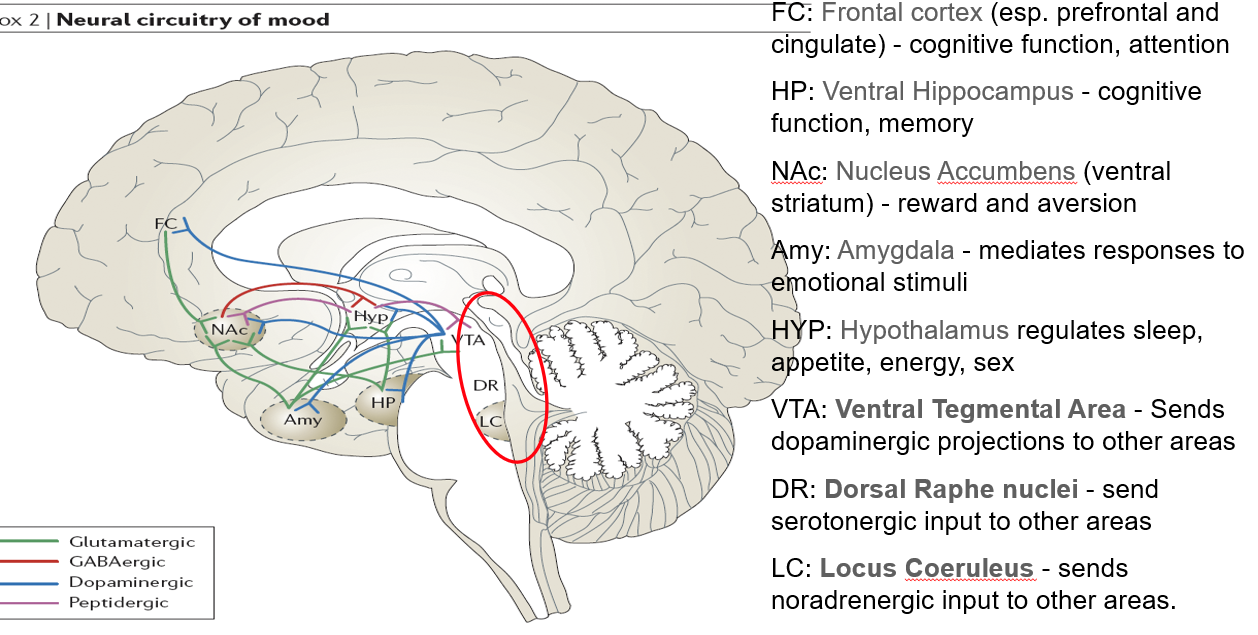
What role does the amygdala play in mood and behavior?
Mediates responses to emotional stimuli
Involved in fear, anxiety, and emotional memory
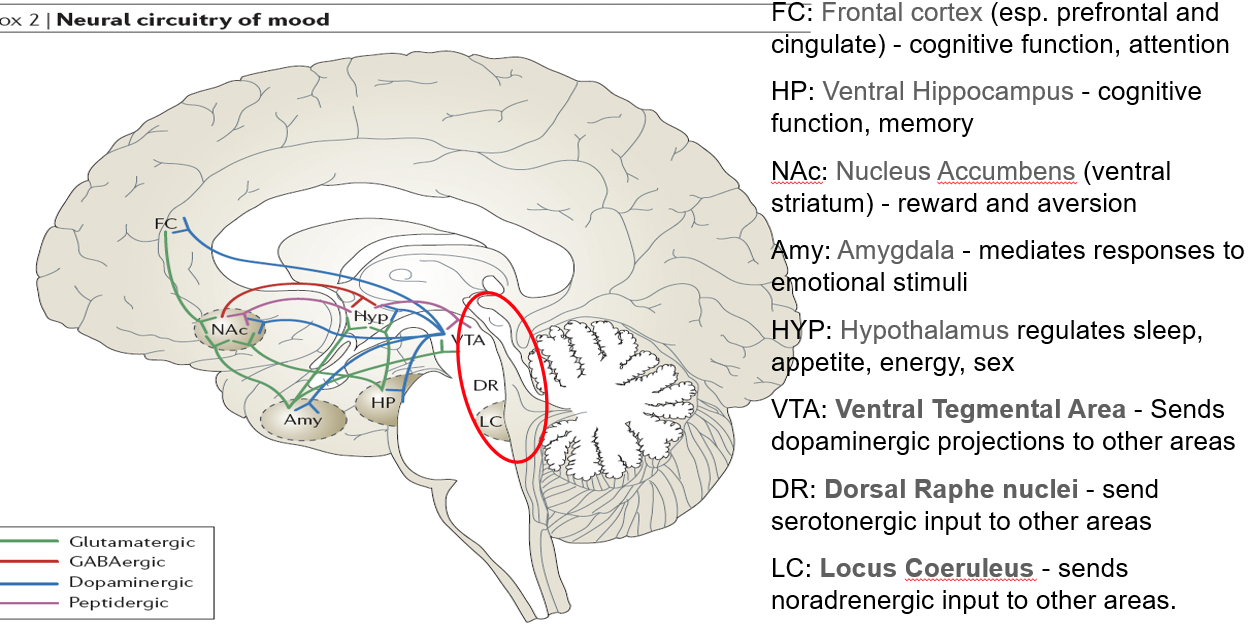
What functions are regulated by the hypothalamus (HYP)?
Controls sleep, appetite, energy balance, and sex drive
Key player in neuroendocrine function
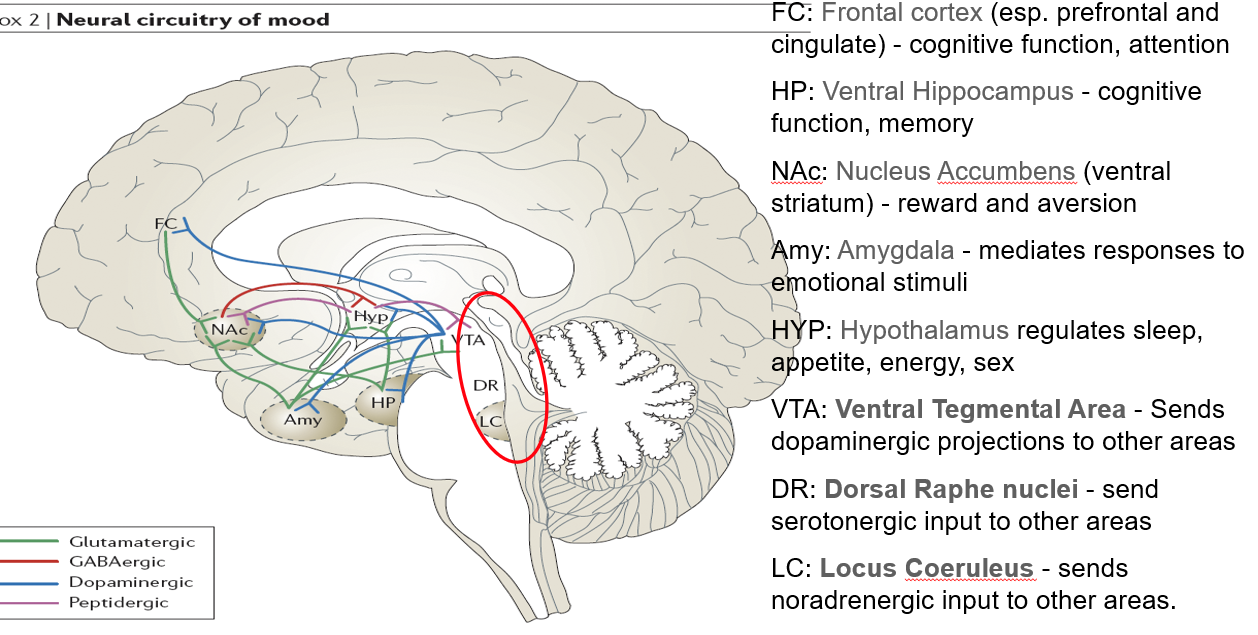
What is the role of the ventral tegmental area (VTA) in neurotransmission?
Sends dopaminergic projections to several brain areas
Central to reward, motivation, and mood regulation
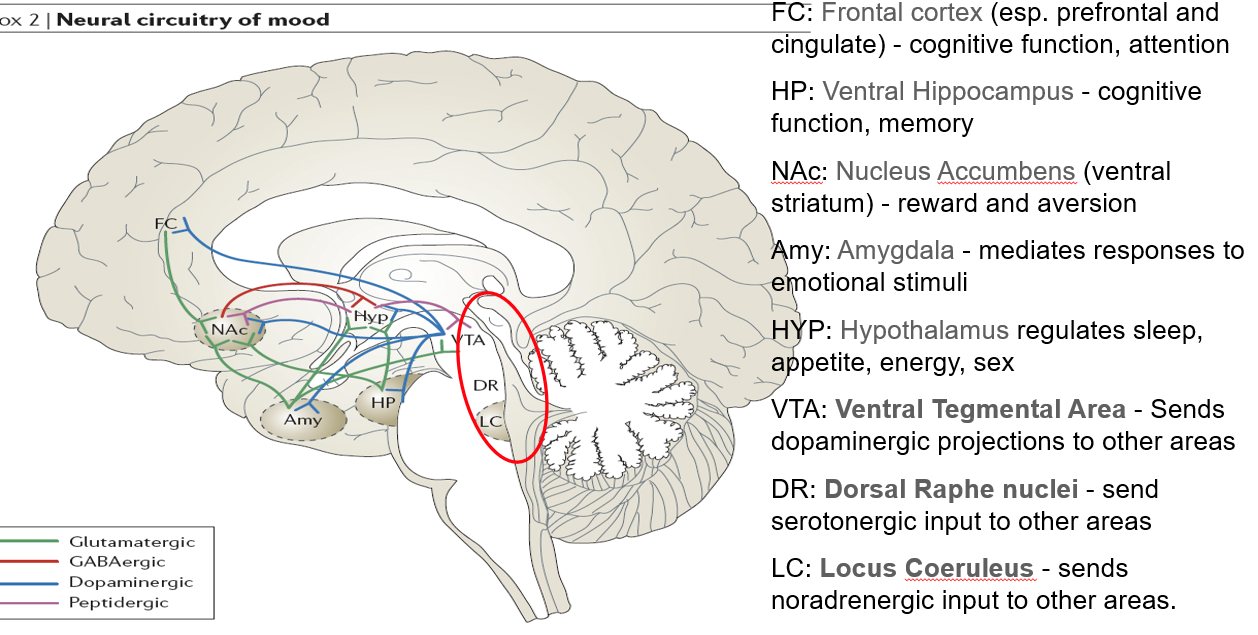
What does the dorsal raphe nuclei (DR) do?
Sends serotonergic (5-HT) input to various brain regions
Major source of serotonin in the brain
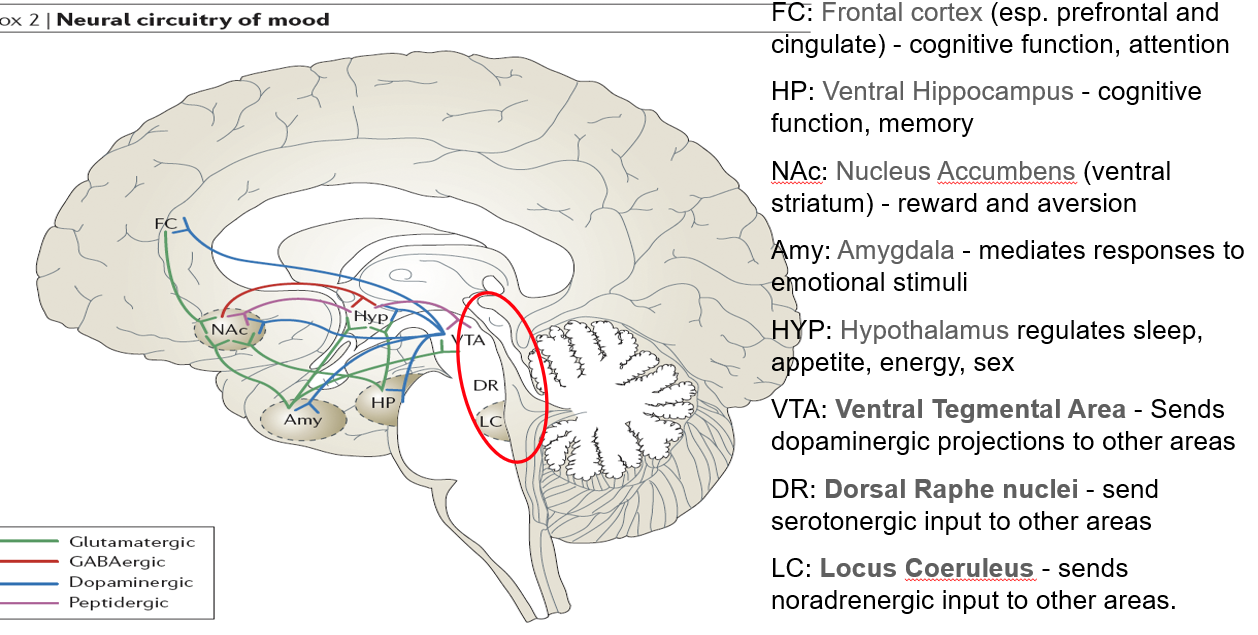
What is the function of the locus coeruleus (LC) in mood and alertness?
Sends noradrenergic (NA) projections throughout the brain
Important in arousal, attention, and stress responses
the stepped care model NIC step 1?
assessment, support psychoeducation, active monitoring and referral for further assessment and interventions.
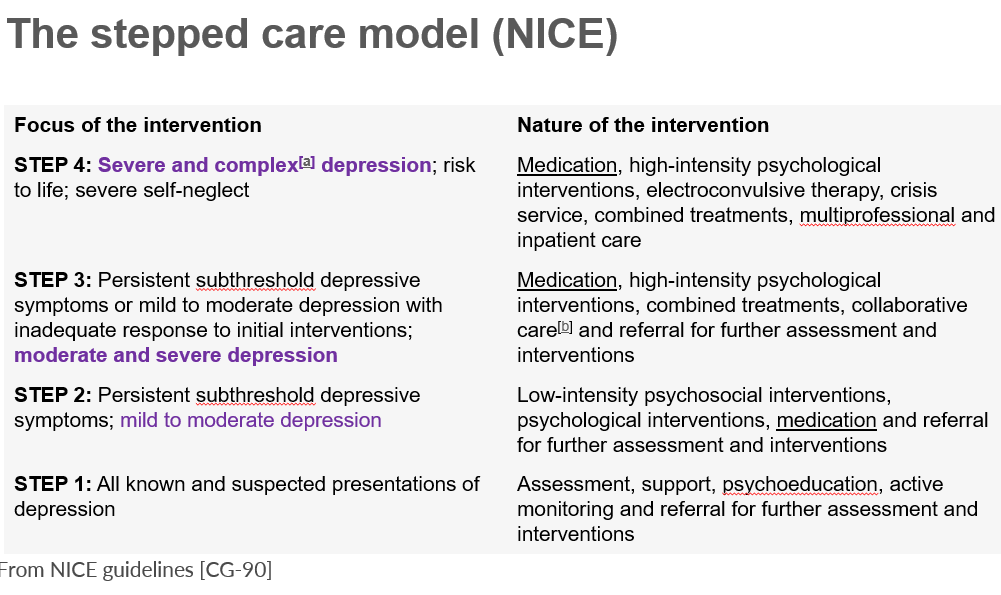
step 2 of the stepped care model NICE
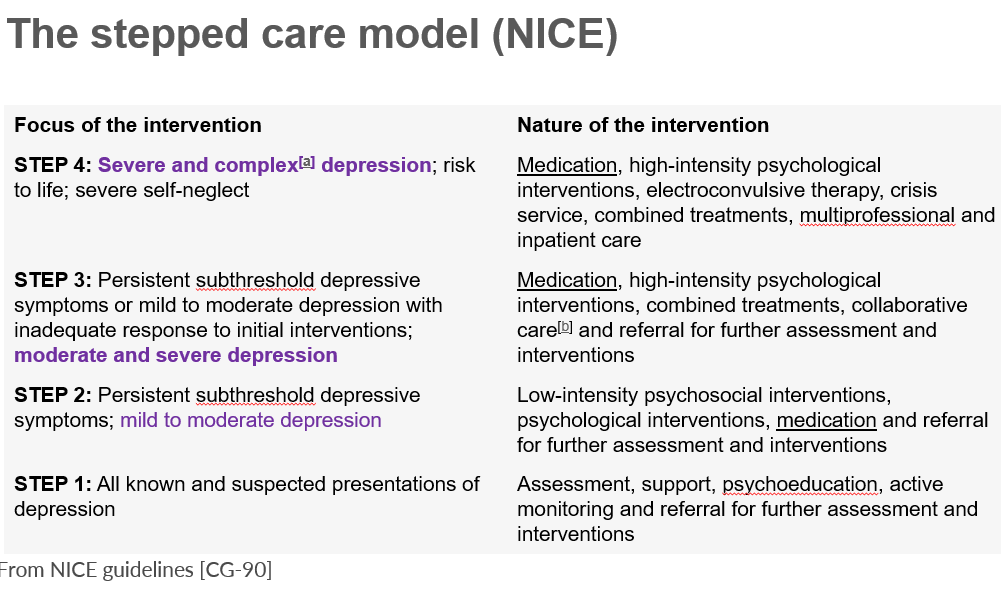
step 3 of the stepped care model NICE
medication
high intensity psychological interventions,
combined treatments
collaborative care
referral for further assessment and interventions
What is Interpersonal Psychotherapy (IPT) and what does it focus on?
A short-term psychodynamic therapy
Focuses on improving current relationships
Aims to reduce symptoms by resolving interpersonal conflicts and life role transitions
What are the main goals of cognitive therapy for mood disorders?
Monitor and identify automatic thoughts
Challenge and replace negative or distorted thinking with more neutral or positive thoughts
Aims to change unhelpful cognitive patterns that maintain depression
What is the goal of behavioral activation in treating depression?
Encourages re-engagement with positive or meaningful activities
Aims to break the cycle of withdrawal and inactivity
Helps improve mood through increased rewarding behavior
: Mindfulness-Based Cognitive Therapy (MBCT)
Designed to prevent relapse in recurrent depression
Combines mindfulness strategies (like meditation) with cognitive techniques
Encourages non-judgmental awareness of thoughts and feelings
what are 3 psychological treatment options of mood disorders?
IPT
cognitive therapy CBT
mindfulness based cognitive therapy MBCT
What is ECT and how does it work in treating depression?
A medical procedure that induces a brain seizure and momentary unconsciousness
Used to rapidly relieve severe symptoms of depression
What are the main clinical situations where ECT is recommended?
ECT is reserved for:
Severe depression with high suicide risk
Depression with psychotic features
Treatment-resistant depression (non-responders to medication/therapy)
What is unilateral ECT and why is it used?
ECT applied to one hemisphere of the brain (usually non-dominant side)
Aims to reduce cognitive side effects, especially memory loss
What is the most common side effect of ECT?
memory loss
what are 3 classes of antidepressants
monoamine oxidase inhibitors
tricyclic antidepressants : 5ht selective ssri’s
receptor blockers
What do MAOIs (e.g. phenelzine, tranylcypromine, iproniazid) do?
Irreversible inhibitors of monoamine oxidase (MAO)
Increase levels of monoamines (e.g. noradrenaline, serotonin) by blocking their breakdown
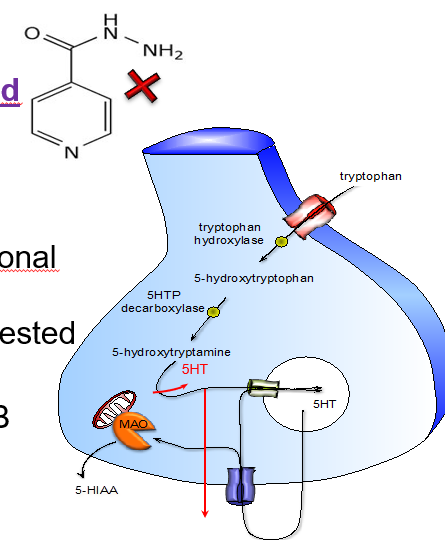
Where is MAO expressed and what is its function?
Widely expressed, especially in:
Nerve terminals: regulates intraneuronal concentration of NA and 5-HT
Gut wall: inactivates endogenous and ingested amines (e.g. tyramine)
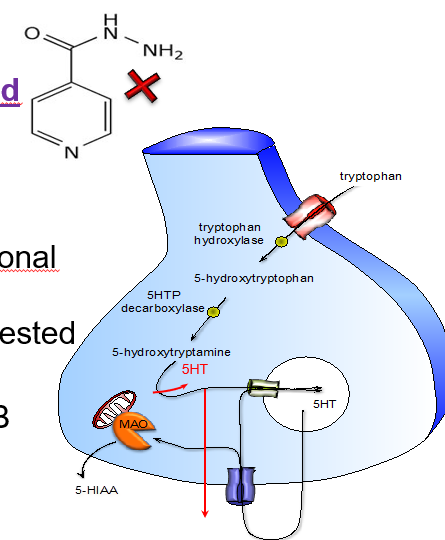
What are the two forms of monoamine oxidase, and what do they preferentially break down?
MAO-A: prefers serotonin (5-HT)
MAO-B: prefers dopamine (DA)
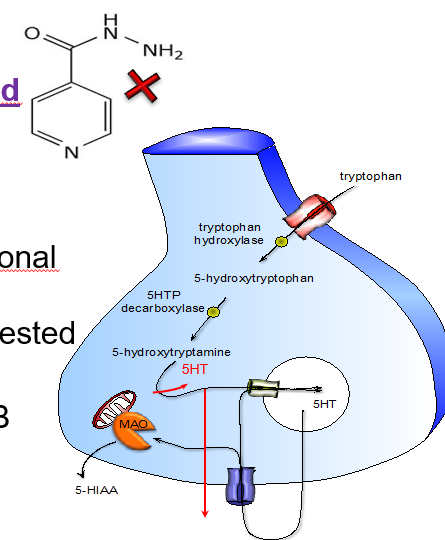
Name 3 common MAOI drugs.
Phenelzine
Tranylcypromine
Iproniazid
What are MAOIs (e.g. phenelzine, tranylcypromine, iproniazid) and how do they affect neurotransmitters?
MAOIs are irreversible inhibitors of the enzyme monoamine oxidase (MAO).
They work by blocking the breakdown of monoamines such as noradrenaline (NA) and serotonin (5-HT), increasing their levels in the brain.
Where is MAO found in the body and what are its functions?
Monoamine oxidase (MAO) is widely expressed in the body:
In nerve terminals, it helps regulate the internal (intraneuronal) levels of 5-HT and NA
In the gut wall, it breaks down endogenous and ingested amines, such as tyramine, preventing toxicity
What are the two types of MAO enzymes, and what neurotransmitters do they target?
There are two forms of the MAO enzyme:
MAO-A primarily breaks down serotonin (5-HT)
MAO-B primarily breaks down dopamine (DA)
What is the "cheese reaction"?
It’s a potentially life-threatening hypertensive crisis triggered when people on non-selective, irreversible MAOIs consume foods high in tyramine (e.g. aged cheese, cured meats).
: What is tyramine, and in which foods is it commonly found?
Tyramine is a naturally occurring amine found in fermented or aged foods, such as:
Aged cheeses
Cured meats
Red wine
Soy products
Pickled or fermented foods
What normally happens to dietary tyramine in the body?
yramine is usually metabolised in the gut and liver by the enzyme monoamine oxidase (MAO), preventing it from entering the bloodstream.
What happens to tyramine metabolism when a person is on a non-selective, irreversible MAOI?
MAO is inhibited, so tyramine is not broken down and instead gets absorbed into the bloodstream, leading to increased noradrenaline release.
What effect does absorbed tyramine have in the body?
It causes a sympathomimetic response — triggering the release of noradrenaline, which can lead to severe hypertension and other complications.
What are the potential symptoms or health risks of the cheese reaction?
acute hypertension
Severe headache
Angina (chest pain)
Cardiac arrest
Pulmonary oedema
Intracranial haemorrhage
Which types of MAOIs are most associated with the cheese reaction?
Non-selective, irreversible MAOIs, such as:
Phenelzine
Tranylcypromine
Iproniazid
What precaution should patients on MAOIs take to avoid the cheese reaction?
They must follow a low-tyramine diet, strictly avoiding aged, fermented, or cured foods.
What is tyrosine, and why is it important in the brain?
tyrosine=amino acid
precursor to neurotransmitters like dopamine, na, adrenaline.
tyrosine also precursor to tyramine.
how is tyramine made in the body?
Tyramine is formed by the decarboxylation of tyrosine via the enzyme aromatic L-amino acid decarboxylas (AADC)
Tyrosine
→ (via decarboxylation)
→ Tyramine
How does the body normally handle tyramine?
Tyramine is broken down by the enzyme monoamine oxidase (MAO) in the gut and liver, preventing it from entering circulation and causing problems.
What happens if someone on a non-selective MAOI consumes tyramine?
Tyramine is not metabolised, gets absorbed, and enters nerve terminals, triggering a massive release of noradrenaline → leading to a hypertensive crisis (cheese reaction).
what are the major side effects of moai’s?
oHypotension (sympathetic block)
oAtropine-like effects
oHepatocellular jaundice
why has there been a decline in use of maoi’s?
concern over safety.
hypertensive crisis
What has caused renewed interest in MAOI treatments?
The development of selective and reversible inhibitors of monoamine oxidase (RIMAs) has renewed interest in MAOIs.
example of a reversible inhibitor of monoamine oxidase A (RIMA)?
Moclobemide
How does moclobemide's reversible action impact its inhibition of MAO-A?
Because moclobemide reversibly inhibits MAO-A, its effect can be partially reversed by high concentrations of substrates like tyramine.
Is moclobemide likely to cause the hypertensive "cheese reaction" when tyramine-containing foods are ingested?
It is unlikely to cause the cheese reaction because its reversible inhibition allows MAO-A activity to resume in the presence of high tyramine levels.
name examples of tricyclic antidepressants (TCAs)? Tricyclic Antidepressants
imipramine, amitriptyline, and clomipramine
What is the primary mechanism of action of TCAs on neurotransmitters?
TCAs block the reuptake of monoamines by nerve terminals, primarily serotonin (5-HT) and noradrenaline (NA), dopamine (DA).
Are TCAs selective in blocking neurotransmitter uptake?
No, TCAs are relatively non-selective and act competitively with endogenous neurotransmitters.
Which receptors do TCAs block that contribute to their side effect profile?
TCAs block muscarinic acetylcholine receptors (mAChRs), histamine receptors (HA), and serotonin receptors (5-HT), which contribute to side effects.
Are the metabolites of TCAs pharmacologically active?
Yes, major metabolites of TCAs are also active and contribute to their effects.
what are the major side effects of tca’s?
•Sedation
•Atropine-like (muscarinic blockade)
•Postural hypotension
•Mania and convulsions (rare)
•Dysrhythmia and heart block
Why does acute overdose limit the clinical use of TCAs?
Because TCAs can cause severe, life-threatening toxicity including antimuscarinic effects, cardiac arrhythmias, CNS depression, and respiratory failure.
What central nervous system symptoms occur in acute TCA overdose?
Symptoms include confusion, mania, delirium, coma, and respiratory depression due to antimuscarinic and monoaminergic effects.
How does TCA overdose affect the heart?
It causes cardiac arrhythmias, conduction block, and hypotension due to sodium channel blockade in cardiac tissue.
How does the toxicity of TCAs affect their clinical use?
TCAs are used with caution, especially in suicidal patients, and are often replaced by safer antidepressants like SSRIs due to their dangerous overdose profile.
What respiratory problems can occur with TCA overdose?
Respiratory depression and hypoxia can result from CNS depression.
what are the drug interactions for tca’s?
•Alcohol
•Hypotensives
•NSAIDs
•MAOIs…..
Can you name some common SSRIs?
Examples include fluoxetine (Prozac), paroxetine, and citalopram.
When were SSRIs developed and what was the rationale behind their development?
SSRIs were developed by Eli Lilly in the 1980s, based on the idea that:
The ‘biological’ components of depression respond to antidepressants affecting noradrenaline (NA) systems.
The ‘emotional’ components respond to antidepressants affecting serotonin (5-HT) systems.
What is the clinical significance of SSRIs in treating depression?
SSRIs are the most commonly prescribed class of antidepressants and are considered first-line treatments for depression.
Besides depression, what other disorders are SSRIs commonly prescribed for?
SSRIs are also widely used to treat anxiety disorders.
How does mirtazapine work?
Mirtazapine blocks α2-adrenoceptors and 5HT2C receptors, leading to enhanced release of noradrenaline (NA) and serotonin (5-HT).
What are the suggested advantages of mirtazapine over other antidepressants?
It may be more rapid acting and have fewer side effects, though this is still under investigation.
How does trazodone work?
Trazodone blocks 5HT2A and 5HT2C receptors and inhibits serotonin reuptake
What is the mechanism of mianserin?
Mianserin blocks multiple 5HT receptors as well as α1 and α2 adrenoceptors.
What is the role of agomelatine in depression treatment?
Agomelatine blocks melatonin receptors and is especially useful for depression associated with sleep disturbances.
What tolerability issue may deter patients from using SSRIs?
Emotional blunting or emotional detachment is a common side effect that can reduce SSRI acceptability.
What is the usual time frame for antidepressants to show therapeutic benefits?
It typically takes 4-6 weeks for antidepressants to produce noticeable therapeutic effects.
What are the major safety concerns associated with antidepressant use?
Risks include increased suicide risk (especially early in treatment) and cardiotoxicity with older antidepressants, which can be dangerous in overdose.
Why are older antidepressants considered more hazardous than newer ones?
Older antidepressants are more cardiotoxic and pose a greater risk of fatal overdose compared to newer agents like SSRIs.
What is the immediate pharmacological effect of MAOIs?
MAOIs increase serotonin (5-HT) and noradrenaline (NA) by inhibiting their metabolism via monoamine oxidase enzymes.
How do TCAs increase monoamine levels?
TCAs increase 5-HT and NA by blocking their reuptake into nerve terminals.
What is the immediate effect of SSRIs on neurotransmitters?
SSRIs increase serotonin (5-HT) by blocking its reuptake, making more serotonin available in the synaptic cleft.
Do the pharmacological effects of antidepressants result in immediate symptom relief?
No, clinical effects usually take several weeks (typically 4–6 weeks) to appear, despite immediate changes in neurotransmitter levels
What does the delay in clinical response to antidepressants suggest about their mechanism?
It suggests that chronic adaptive changes (e.g., receptor regulation, gene expression) are required for therapeutic effects, rather than just acute monoamine increases.
what are the medications for bipolar disorder? AKA mood stabilisers?
Lithium
•Up to 80% receive at least some relief with this mood stabilizer
•Potentially serious side effect = Lithium toxicity
Anticonvulsants (prevent relapse to depression?)
•e.g. valproate, lamotrigine
Antipsychotics (co-prescribed, where mania predominates?)
•e.g. olanzapine, risperidone, quetiapine, aripiprazole
clinical utility of lithium, what are they if they are mainly used prophylactically?
oOnly reduce mania in acute episodes
oReduce both depressive and manic phases
oLong term treatment to prevent relapse
oEffects seen after 3-4 weeks of treatment
clinical utility of lithium: narrow therapeutic window.
what concentration is potentially fatal for someone with bipolar disorder?
oTherapeutic range; 0.4 mmol/L – 1.0 mmol/L
oPotentially fatal > 1.5mmol/L
oPlasma concentration monitoring required
what are the acute side effects of lithium?
•Polyuria and polydipsia (renal problems)
•Weight gain
•Aggravation/precipitation of skin disorders
•Tremor,
Muscle twitching, Dysarthria
•Nausea,
Vomiting,
Appetite loss,
Diarrhoea
•Drowsiness
•Coma,
•Choreiform and Parkinsonian motor symptoms
•Seizure and Convulsions
what are the chronic symptoms of lithium use?
•Thyroid goitre
•Nephrotoxicity
•Hair loss
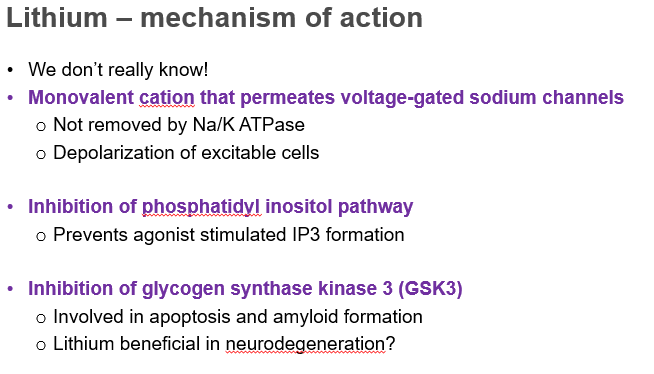
lithium mechanism of action
we dont really know.
slide 52 on lecture.