Proton transfer reactions (acids and bases)
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
bronsted lowry acids and bases
bronsted lowry acid: gives away a proton
bronsted lowry base: accepts a proton using its lone pair of electrons
conjugate acids and bases
conjugate base of an acid: species remaining after the acid donates a proton
conjugate acid of a base: species remaining after the base accepts a proton
amphiprotic species
species that can act as both proton donors and proton acceptors
e.g water
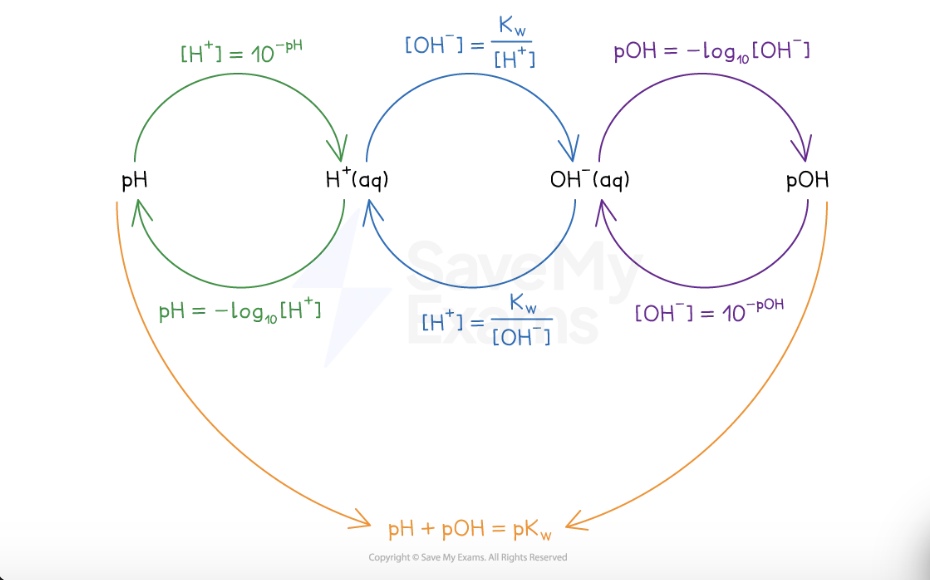
the pH scale
the acidity of an aqueous solution depends on the number of H+ ions in the solution
pH = – log10[H+]
[H+] = 10–pH
pH usually given to 2 d.p
logarithmic scale, each pH value is 10 times the value below it
the lower pH, the more acidic, the higher pH, the more alkaline
pH of acids, bases and water explained
acidic solutions
always have more H+ than OH- ions
[H+] always greater than 10-7 mol dm-3, so pH always below 7
basic solutions
always have more OH- ions than H+ ions
[H+] is always smaller than 10-7 mol dm-3, so pH always above 7
pH of water
at 298K, water has equal amounts of OH- and H+ ions with concs of 10-7 mol dm-3
how to measure pH
pH meter:
most accurate
connected to pH electrode which shows the pH value of solution
universal indicator
less accurate
dipped into solution of acid and changes colour
colour is compared to a chart which shows different pH values
the ion product of water (Kw)
an equilibrium exists in water, where a few water molecules dissociate into H+ and OH- ions
H2O (l) ⇌ H+ (aq) + OH– (aq)
equilibrium constant for this reaction is:
Kc = [H+][OH-] / [H2O]
Kc x [H2O] = [H+][OH-]
since the conc of H+ and OH- ions is very small, conc of water is considered to be a constant
so Kw = [H+] [OH-]
Kw (ion product of water) = Kc x [H2O] = 1.00 x 10-14 at 298K
how does temp affect Kw
ionisation of water is an endothermic process, so due to le chateliers principle, an increase in temp will favour the forward reaction
[H+][OH-] increases
magnitude of Kw increases
pH decreases
increasing temp decreases pH of water (more acidic)
decrease temp increases pH of water (more basic)
strong and weak acids
strong acid: dissociates almost completely in aqueous solutions
position of equilibrium extremely right, irreversible
e.g HCl, HBR, HI, HNO3, H2SO4
solution formed is highly acidic due to high conc of H+ ions
pH can be calculated if concentration of strong acid is known
weak acids: acids that partially dissociate in aqueous solutions
position of equilibrium is more to the left equilibrium is established (reversible reaction)
e.g ethanoic acid
solution formed is less acidic due to lower concentration of H+ ions
strength of bronsted lowry acid depends on the ease with which it dissociates to released H+ ions
strong and weak bases
strong base: base that dissociates almost completely in aqueous solution
position of equilibrium extremely right, irreversible
e.g NaOH
solution formed highly basic due to high conc of OH- ions
weak base: base that partially dissociates in aqueous solution
position of equilibrium more left, equilibrium is established (reversible)
e.g NH3
solution formed less basic due to lower conc of OH- ions
strength of conjugate acids and bases
strong acids produce weak conjugate bases
because reverse reaction is virtually non existent
weak acids produce strong conjugate bases
strong bases produce weak conjugate acids
weak bases produce strong conjugate acids
distinction between strong and weak acid
pH value
the stronger the acid, the greater the H+ conc, the lower the pH
electrical conductivity
stronger acids give higher readings conductivity meter because they have more ions in solution
reactivity
as conc of H+ is much greater in strong acids the rate of reaction with metals and metal compounds is greater than of weak acids of the same conc
neutralisation reaction
acid + base —> salt + water
H+ + OH- —> H2O
spectator ions not involved in formation of water form the the salt
summary of neutralisation reactions
acid + metal —> salt + hydrogen gas
extent of reaction depends on reactivity of metal and strength of acid
acid + metal oxide —> salt + water
acid + metal carbonate —> salt + water + carbon dioxide
acid + metal hydrogencarbonate —> salt + water + carbon dioxide
SA + SB pH curve
e.g NaOH + HCl
shows how the pH of a solution changes as the acid/base is added in a strong acid/strong base titration
pH of acid: where the curve starts on y-axis, roughly 1
equivalence point: “stoichiometric end of reaction”, when the acid/base neutralisation is complete
calculating pH after x volume of base added:
calculate moles of acid and base (conc x volume of base added)
determine moles of excess acid
determine new volume (vol of acid + added volume of base)
[H+]= moles of excess acid / new volume
-log[H+] = pH
![<ul><li><p>e.g NaOH + HCl</p></li><li><p>shows how the pH of a solution changes as the acid/base is added in a strong acid/strong base titration</p><ul><li><p>pH of acid: where the curve starts on y-axis, roughly 1</p></li><li><p>equivalence point: “stoichiometric end of reaction”, when the acid/base neutralisation is complete</p></li></ul></li><li><p>calculating pH after x volume of base added:</p><ol><li><p>calculate moles of acid and base (conc x volume of base added)</p></li><li><p>determine moles of excess acid</p></li><li><p>determine new volume (vol of acid + added volume of base)</p></li><li><p>[H+]= moles of excess acid / new volume</p></li><li><p>-log[H+] = pH</p></li></ol></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/5a6e3bd7-fe94-42e3-89d9-704b05d91c18.png)
pOH scale
basicity of aqueous solution depends on number of hydroxide ions
pOH = -log [OH-]
pH = 14 - pOH
relationship between H+ OH- pH and pOH
dissociation of a weak acid
weak acids HA dissociate partially in water
HA (aq) ⇌ A- (aq) + H+ (aq)
at equilibrium, majority of HA molecules remain unreacted
position of equilibrium is more left, equilibrium is established
acid dissociation constant, Ka = [A-][H+] / [HA]
values of Ka are very small
dissociation of a weak base
weak bases (B) ionise in water
B (aq) + H2O (l) ⇌ BH+ (aq) + OH- (aq)
equilibrium is established
base dissociation constant Kb = [BH+][OH-] / [B]
assumptions made when calculating pKb, Kb, pKa, Ka
initial conc of acid is about the equilibrium conc of acid
[A-] = [H+]
negligible ionisation of the water, so [H+] is not affected
The temperature is 298 K
pKa and pKb
pKa = -logKa
pKb = -logKb
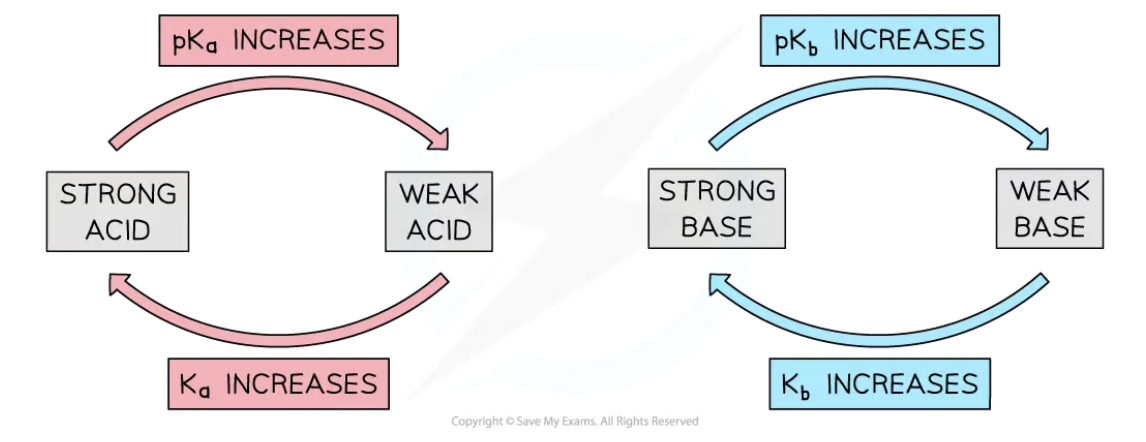
Ka and Kb of conjugate acid-base pairs
Ka x Kb = Kw
salt hydrolysis
ionic salt is formed from neutralisation of acid and base
HA (parent acid) + MOH (parent base) —> M+A- (salt) + H2O
ionic salt formed will dissociate in water
hydrolysis is where water is used to break a bond within compound, resulting in aqueous ions
pH of salt depends on the strength of parent acid and base used
summary of salt hydrolysis reactions
SA + SB
neutral
weak conjugate acid and base ions therefore no hydrolysis occurs
equivalence point= pH 7
SA + WB
acidic
strong conjugate acid of WB is formed, which reacts with water to form H+ ions, therefore solution becomes more acidic
M+ + H2O —> MOH + H+
equivalence point = <7 pH
WA + SB
alkaline
strong conjugate base of WA is formed, which reacts with water to form OH- ions, therefore solution becomes more basic
A- + H2O —> HA + OH-
equivalence point = >7 pH
WA + WB
depends on relative Ka and Kb
WA + SB pH curve
e.g NaOH + CH3COOH
starting pH roughly 3 because WA
initial rise in pH steep as neutralisation of WA by strong base is rapid
ethanoate ions (conjugate base to weak acid) are formed, creating a buffer
buffer formed will resist changes in pH so pH rises gradually in buffer region
pKa = pH [H+] = Ka at half equivalence point
equivalence point = >7 pH
![<ul><li><p>e.g NaOH + CH3COOH</p></li><li><p>starting pH roughly 3 because WA</p></li><li><p>initial rise in pH steep as neutralisation of WA by strong base is rapid</p></li><li><p>ethanoate ions (conjugate base to weak acid) are formed, creating a buffer</p></li><li><p>buffer formed will resist changes in pH so pH rises gradually in buffer region</p></li><li><p>pKa = pH [H+] = Ka at half equivalence point</p></li><li><p>equivalence point = >7 pH</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c6a16091-93e7-444b-aeed-ddc4f3be66e4.png)
SA + WB pH curve
e.g NH3 + HCl
starting pH roughly 11 (WB)
pH falls as WB begins to be neutralised and forms conjugate acid
creates buffer region so pH falls slowly
half equivalence point (where half the amnt of WB has been neutralised)
pKb = pOH [OH-] = Kb at half equivalence point
equivalence point = <7 pH
acid-base indicators
weak acid which dissociates to make anion of a different colour
e. g HInd ⇌ H+ (aq) + Ind– (aq)
HIn and its conjugate base Ind- are different colours
if solution acidic, equilibrium shifts left and HInd colour is shown
if solution basic, equilibrium shifts right, and Ind- colour is shown
universal indicator is a mix of many indicators with a wide pH range of colour
indicator calculations
pH at which transitions between colours occur:
Ka
endpoint of reaction is when there is balance of [HIn] and [In-]
Ka = [H+][Ind-] / [HInd] = [H+]
take negative logs of both sides
pKa = pH
pKa of an indicator is the same as its pH at end point
colour change takes place over range of pH = pKa ± 1
choosing the right indicator
indicator is appropriate if it has an endpoint range that coincides with pH at equivalence point
buffer solutions
solution which resists changes in pH when small amounts of acid/base are added
used to keep the pH almost constant
acidic buffers
consists of weak acid, the salt of that acid, and a strong base
e.g aqueous mixture of sodium ethanoate and ethanoic acid
ethanoic acid is weak, ionises in solution to form relatively low conc of ethanoate ions
sodium ethanaote is a salt, fully ionises in solution forming high conc of ethanoate ions
contains reserves supplies of the acid and its conjugate base
high conc of ethanoic acid due to its partial ionisation
high conc of ethanoate ions due to full ionisation of sodium ethanaote
in buffer solution, ethanoic acid in equilibrium with hydrogen and ethanoate ions
adding H+ to acidic buffer
H+ reacts with the ethanoate ions, forming undissociated ethanoic acid
СН3COO- (aq) + H+(aq) = СН3СООН (аq)
pH remains constant
adding OH- to acidic buffer
react with the undissociated acid (ethanoic acid) to produce H2O
CH3COOH(aq) + OH (aq) → CH3COO- (aq) + H2O
pH remains constant
basic buffers
made by mixing a solution of a weak base with its salt
e.g NH3 and NH4Cl
ammonia partially dissociate in water forming NH4+ and OH-
NH4Cl fully dissociates forming NH4+ and Cl- ions
adding acid/base will result in no change in pH
additional H+ will combine with OH- ions to make water
additional OH- will combine with NH4+ to form NH3
buffer calculations
to determine pH of buffer:
[H+] needed, found using:
[H+]= Ka [acid]/[salt]
pH = pKa + log[salt] /[ acid]
to determine pOH
[OH-] needed, found using
[OH-] =Kb [base] / [salt]
pOH = pKb + log[salt] / [base]
factors affecting buffers
dilution
Ka and Kb are equilibrium constants so not changed by dilution
doesn’t change ratio of acid/base conc to salt conc
temperature
constant temp must be maintained when using buffers
temp affects values of Ka and Kb