reducing unwanted energy transfer
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Give 4 methods of reducing heat loss from a home
-Have thick balls that are made up from a material with a low conductivity
-use cavity walls with air gap
-double glazed windows
-insulate walls and loft
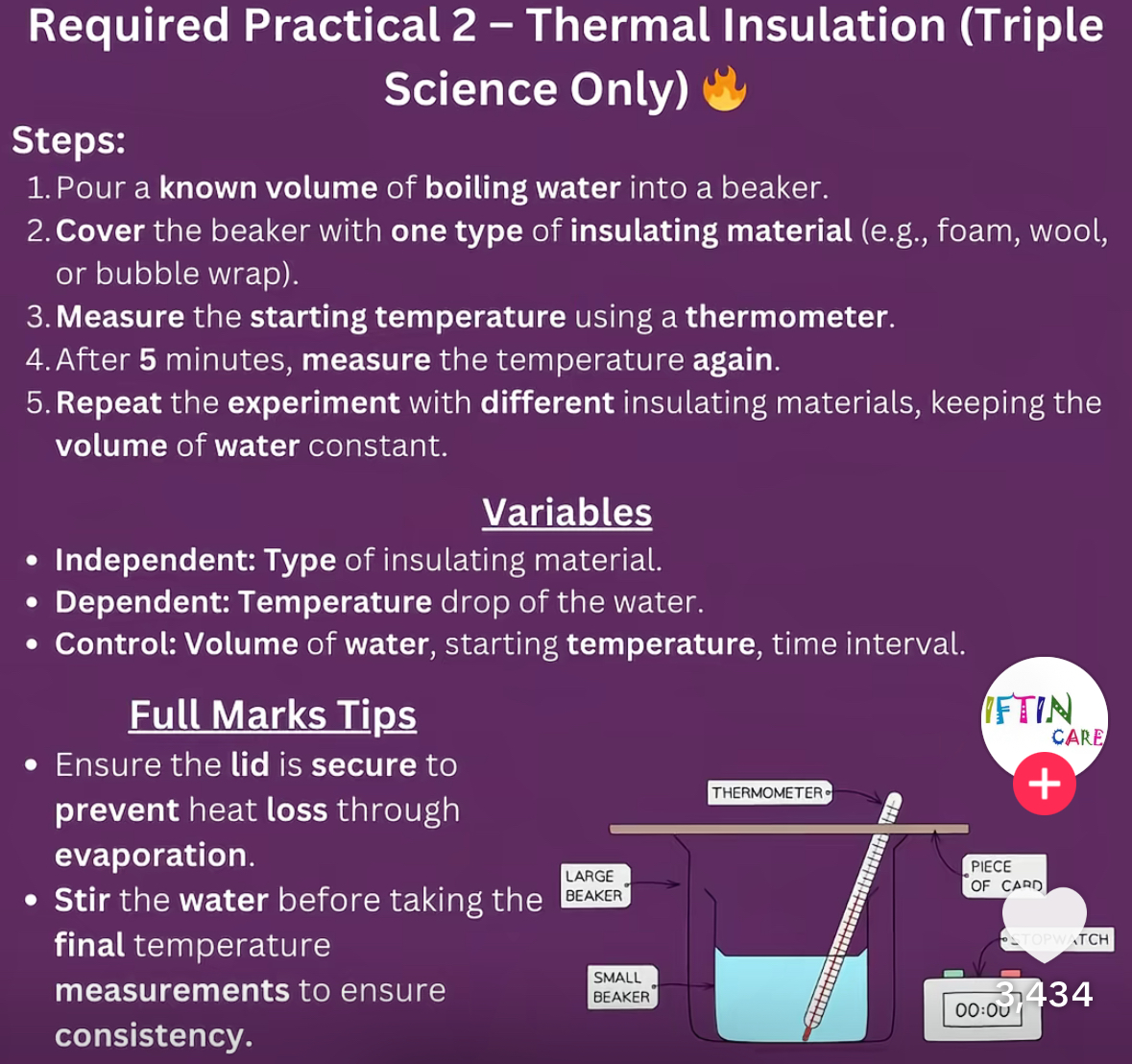
Describe the thermal insulation practical
What is the purpose of putting insulation in the gap in cavity walls?
To minimise convection currents
Air Allows convection by adding the insulating foam with lots of isolated bubbles the gap isn’t quite solid or gas so hardly any conduction/convection
How does the gap in a cavity wall help reduce heat loss?
Air is a poor conductor
Heat cannot be conducted across the gap, as the gap contains mostly air which does not conduct heat (remember that insulation is mostly made of air gaps).
What is the difference between a single glazed and double glazed window?
Single glazed windows only have one pane of glass, whereas double glazed windows have two panes of glass, with a small air gap between them.
Which reduces convection
What is the key benefit of double-glazed windows?
The air gap between the two panes minimises heat loss by conduction. And conduction because the gap between the pains of glass is air which is a poor conductor so conduction can take place but also convection is prevented because they’re a very small amount of air preventing my proper air circulation which doesn’t allow connection current to property form.
What is friction?
Friction is the resistance that an object encounters when moving over a solid, or moving through a liquid.
How could you reduce the friction between a bike chain and the bike cogs?
Add a lubricant, such as oil.
Do double glazed windows have a higher or lower thermal conductivity than single glazed windows?
Double glazed windows have lower thermal conductivity, meaning less thermal energy transfers through them.
What does friction reduce
The efficiency of energy transfer and can cause objects to heat up
Four objects that are being robbed together What can u do to Reduce the friction between the objects surfaces when they move?
use lubricants
Lubricants are usually liquids (like oil)so they can flow easily between objects and coat them
Describe the practical where you investigate the effectiveness of different insulators and investigate energy transfers?
– Boil water and a kettle. Pour some of the water into a sealable container to a safe level. Measure the mass of the water in the container.
– Use the thermometer to measure the initial temperature of the water.
– Seal the container and leave it for five minutes measure this time using a stopwatch
– Remove the lid and measure the final temperature of the water
– Pour away the water and allow the container to cool to room temperature
– Repeat this experiment but wrap the container in a different material (e.g. for your newspaper) once it has been sealed. Make sure you use the same mass of water each time.