A2 Chemistry - Topic 1: Electrochemistry
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
reactions that involve the transfer of electrons
what is a redox reaction
how easily an ion is discharged during electrolysis
what does the relative (Eꝋ ) electrode potential of ions tell you
gets reduced at the cathode
where does the positively charged cation/the more positive electrode potential half cell value get discharged
gets oxidised at the anode
where does the negatively charged anion/the more negative electrode potential half cell value get discharged
cathode - hydrogen forms unless metal is less reactive than hydrogen in the reactivity series, then that metal forms instead
anode - oxygen forms
what forms at the cathode and anode during the electrolysis of an aqueous solution
cathode - cation/metal
anode - anion/non-metal
what forms at the cathode and anode during the electrolysis of a molten solution
cathode - hydrogen will form unless metal is less reactive than hydrogen, then that metal will form
anode - is negative ion is a halide, then halogen gas will form, otherwise oxygen will form
what forms at the cathode and anode during the electrolysis of a concentrated aqueous solution
the amount of substance that is formed at an electrode during electrolysis is proportional to
the amount of time where a constant current to pass
the amount of charge, in coulombs, that passes through the electrolyte (strength of electric current)
what does Faraday’s Law state
Q = Ixt
what is the formula for charge
96500 Cmol^-1
what is the value of Faraday’s constant
the amount of electric charge carried by 1 mole of electrons or 1 mole of singly charged ions
what does Faraday’s unit tell us
F = L x e
F → faraday’s constant (96500 Cmol^-1)
L → Avogadro’s constant (6.02 × 10²³ mol^-1)
e → charge on an electron
what is the formula for the relationship between Faraday’s constant and the Avogradro constant
the voltage when a standard half-cell is connected to a standard hydrogen cell under standard conditions
define the standard electrode potential
the potential difference between two half cells/two electrodes in a cell under standard conditions of 1 atm, 298K, and all solutions being 1 moldm^-3
define standard cell potential
1 atm
298K
1 moldm^-3 solution
what are standard conditions
typically a strip of filter paper soaked in a saturated solution of potassium nitrate of potassium chloride (as nitrates and chlorides are usually soluble)
a salt bridge has mobile ions that complete the circuit
salt bridge ensures that no precipitates form which can affect the equilibrium position of the half cells
what is a salt bridge and what does it do
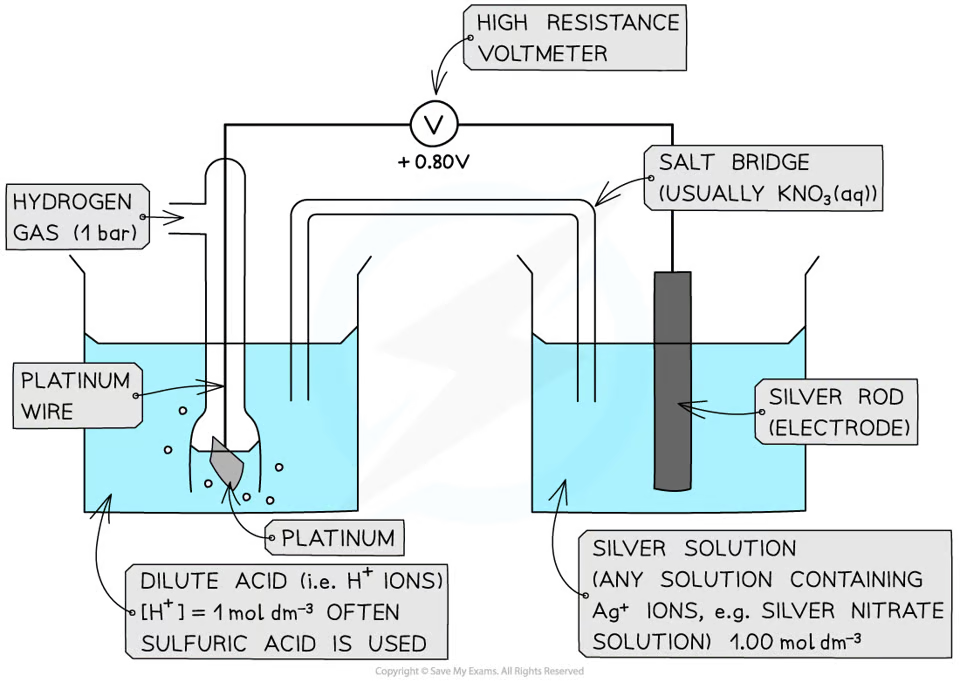
how does a metal/metal ion half cell look like
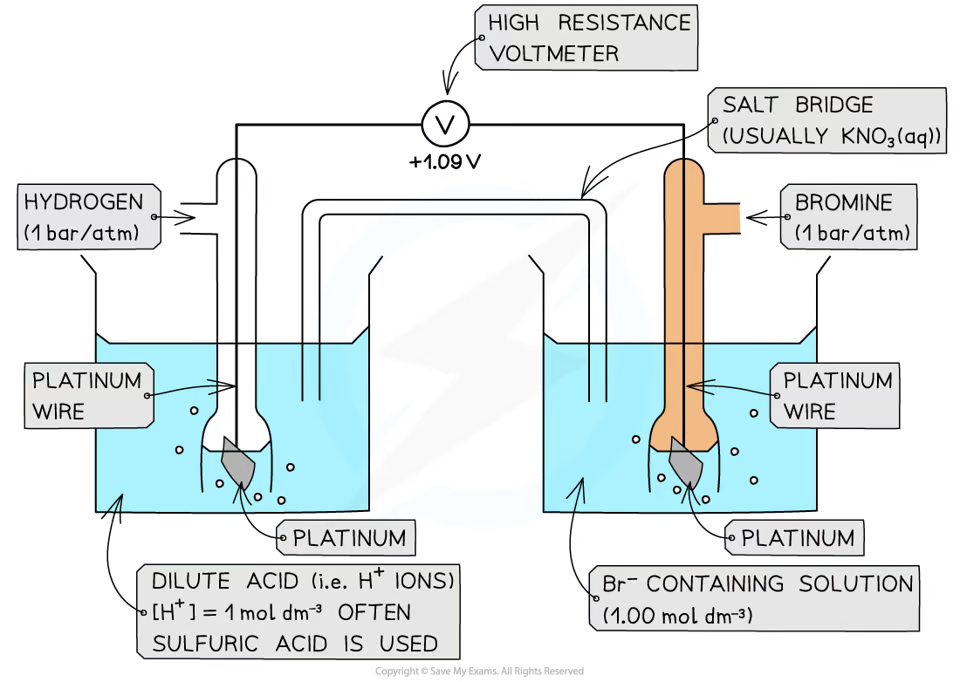
how does a non-metal/non-metal ion half cell look like
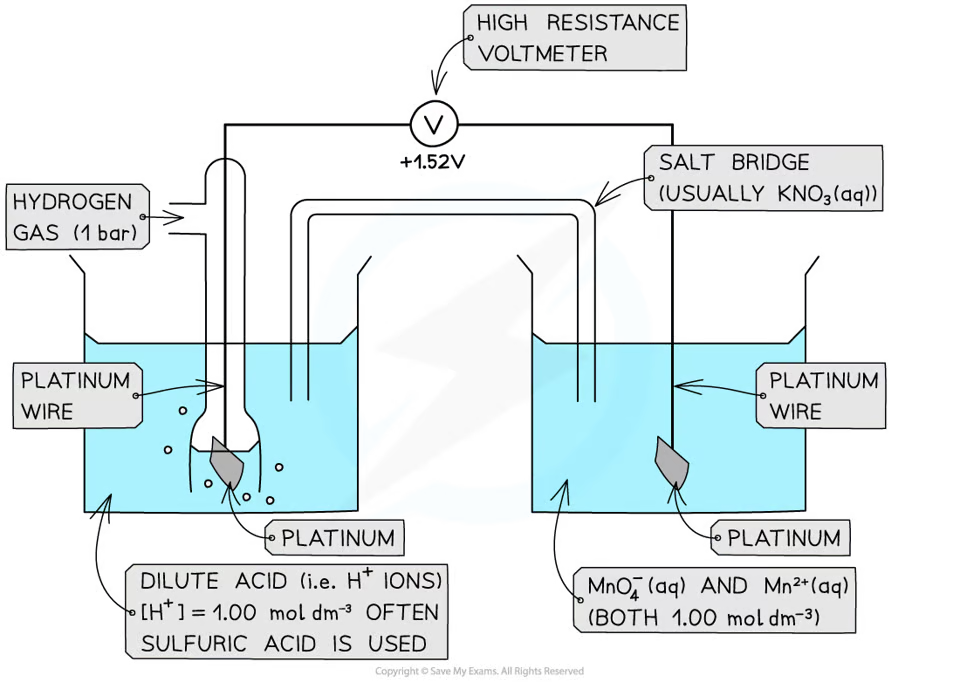
how does a ion/ion half cell look like
the positive pole can more readily accept electrons from the other half cell so gets more readily reduced
the negative pole more readily loses electrons to the other half cell so gets more readily oxidised
the flow of electrons is from the negative pole to the positive pole
how can the direction of electron flow be determined by comparing Ecell values of two half cells in an electrochemical cell
The Ecell values of a species indicate how easily they can get oxidised or reduced
the more positive the value, the easier it is to reduce the species on the left of the half-equation - the reaction will tend to proceed in the forward direction
the less positive the value, the easier it is to oxidise the species on the right of the half-equation, the reaction will tend to proceed in the backward direction
a reaction is feasible (likely to occur) when the Ecellꝋ is positive
predict the feasibility of a reaction using standard cell potentials
if the concentration of the species on the left is increased, the position of equilibrium will shift to the right
this means that the species on the left gets more easily reduced
the Evalue becomes more positive (or less negative)
what is the effect of increasing the concentration of the species on the left on the electrode potential
equilibrium shifts to the left
species on the left gets less easily reduced
Evalue becomes less positive (or more negative)
what is the effect of increasing the concentration of the species on the right on the electrode potential
E = Eθ+ 0.059/z log [oxidised species]/[reduced species]
E - electrode potential under nonstandard conditions
Eθ - standard electrode potential
z - number of electrons transferred in the reaction
The Nernst equation only depends on aqueous ions and not solids or gases
the concentrations of solids and gases are therefore set to 1 moldm^-3
what is the Nernst equation
ΔGꝋ = - n x Ecellꝋ x F
ΔGꝋ - standard Gibbs free energy
n - number of electrons transferred in the reaction
Ecellꝋ - standard cell potential (V)
F - Faraday constant
what is the formula to calculate free energy change using standard electrode potentials