BIO SCI 37 Midterm 2
1/143
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
What is the most significant risk factor for AD?
Age
What is the leading cause of dementia?
Alzheimer’s disorder
Normal aging vs Dementia
Dementia has more frequent/extreme/long-term forgetting
Dementia has changes in mood
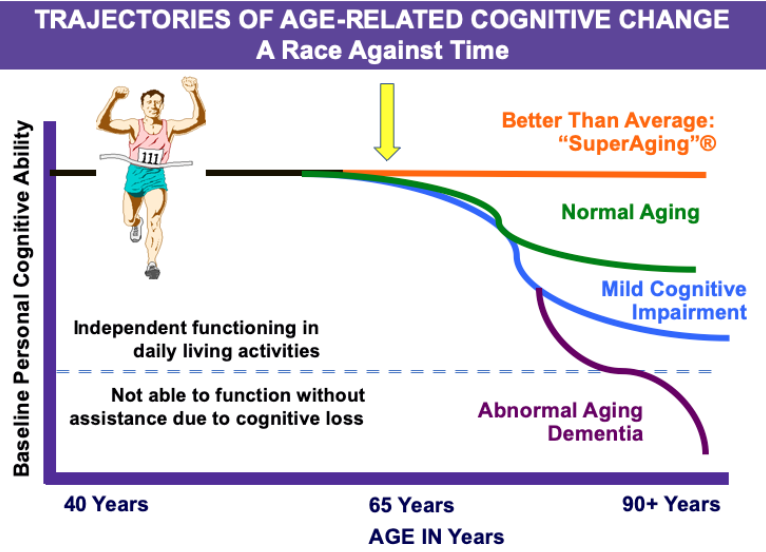
Trajectories of Age-Related Cognitive Change
“Superaging”
Normal aging
Mild cognitive impairment
Abnormal aging dementia
What is “middle aging”
A period of time that shapes future cognitive trajectories and brain health. D
Different types of middle aging
Turning point
Plateau
Breakpoint
Loss of cyclicity
How does brain matter change with age?
Less gray matter = less neurons
Less white matter = thinner myelin sheaths
Synaptic plasticity
The ability of synapses to strengthen or weaken in response to activity
Often associated with structural changes
Symptoms of cognitive decline in aging brains
Slower reaction times
Lower attention level
Slower processing speeds
Decreased sensory and perceptual function
Changes in sleep pattern
Factors that influence brain aging
Genetics
Environment (ie education)
8 hours of sleep per night
The gut’s microbiome (sends signals to the brain)
Inflammation
Neurogenesis
The process by which neurons are generated from neural stem cell and progenitor cells
Progenitor cell
A rudimentary cell, can specialize to become any cell (ie heart cell, liver cell, neuron)
Adult neurogenesis
Neurons generated from adult stem cells
In which areas of the adult brain does neurogenesis occur?
Hippocampus (sub-granular zone)
Sub-ventricular zone
Sub-granular zone
Lead cells to repopulate the dentate gyrus
Sub-ventricular zone
Leads cells to repopulate olfactory bulb
Factors that influence neurogenesis
Increased neurogenesis
Environmental Enrichment
Exercise
Decreased neurogenesis
Neurodegenerative disease
Depression
Aging
Things that delay cognitive decline
Minimized stress, exercise, social life, diet, education
Parabiosis
Greek - “leaving beside”
The anatomical joining of two individuals artificially in physiological research
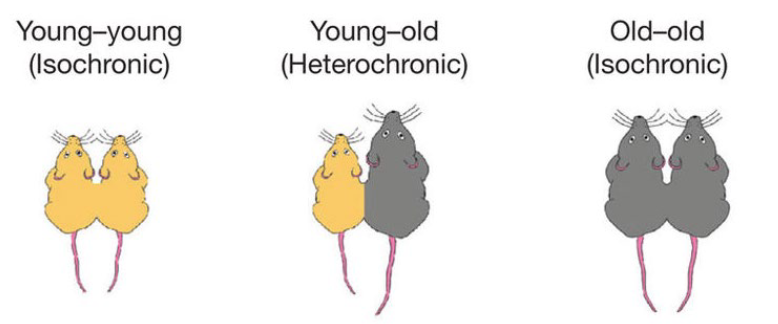
Parabiosis research findings
In young-old connections, the old mouse starts to show similar bodily and brain function as the younger mouse due to the shared blood, so old mice had better cognitive function. Younger mice had worse cognitive function
Types of brain disorders
Specific disorders (focal damage)
Generalized disorders (widespread damage)
Specific disorders
Focal damage
The disorder depends on the area of the brain affected (bullet wounds, strokes, Phineas Gage)
Generalized disorders
Widespread damage
The disorder affects multiple cognitive abilities (closed head injury, dementia, demyelination, toxic substances)
Mild dementing diseases
Person retains judgement and can sustain daily activities on his/her own (personal hygiene etc) but work and social activities are impaired
Moderate dementing disorders
Independent living becomes hazardous (forgets to turn off stove, etc) and requires some degree of supervision
Severe dementing diseases
Cognitive abilities are so impaired that the person requires constant supervision
Aphasia
Loss ability to understand or express speech
Apraxia
Inability to link skilled motor movements to ideas or representations
Agnosia
Deficit in recognizing objects that occurs in the absence of deficits in sensory processing
Acalculia
The inability to perform simple mathematic calculations the patient previously knew
Clinical classifications of types of dementias
Cortical
Subcortical
Mixed
Cortical dementias
Co-occurence of many cognitive deficits including aphasia, apraxia, agnosia, acalculia, visuospatial deficits and memory problems (Alzheimer’s, Frontotemporal dementias, Creutzfeldt-Jakob)
Subcortical dementias
More likely to manifest first as personality changes, attention deficits, slowness in cognitive processing, difficulties with tasks requiring strategy (e.g. Parkinson’s, Huntington’s).
Mixed dementias
Both cortical and subcortical involvement, patterns of cognitive performance midway between cortical and subcortical types (e.g. Vascular dementia, Lewy body dementia.)
What is one of the main criterion of dementia classification?
Underlying pathologies, largely defined by accumulation of abnormal protein aggregates in the brain
What are the 6 main categories of neurodegenerative proteinopathies?
Amyloid-β (Aβ)
Microtubule-associated protein tau
TAR DNA-binding protein 43 (TDP-43)
Fused in sarcoma (FUS)
α-synuclein
Prion protein
Most non-vascular dementias are within one of these 6
Major diseases of dementia
Alzheimer’s Disease
Vascular Dementia
Dementia with Lewy Bodies
Frontotemporal Dementia
Parkinson’s Disease
(Huntington’s Disease)
How are proteins involved in neurodegenerative diseases?
Abnormal protein deposits/protein misfolding leads to problems
What features are present in an Alzheimer’s brain?
Neurofibrillary tangles
Amyloid plaque
Cortical atrophy
Inflammation
Senile Plaques
Neurofibrillary
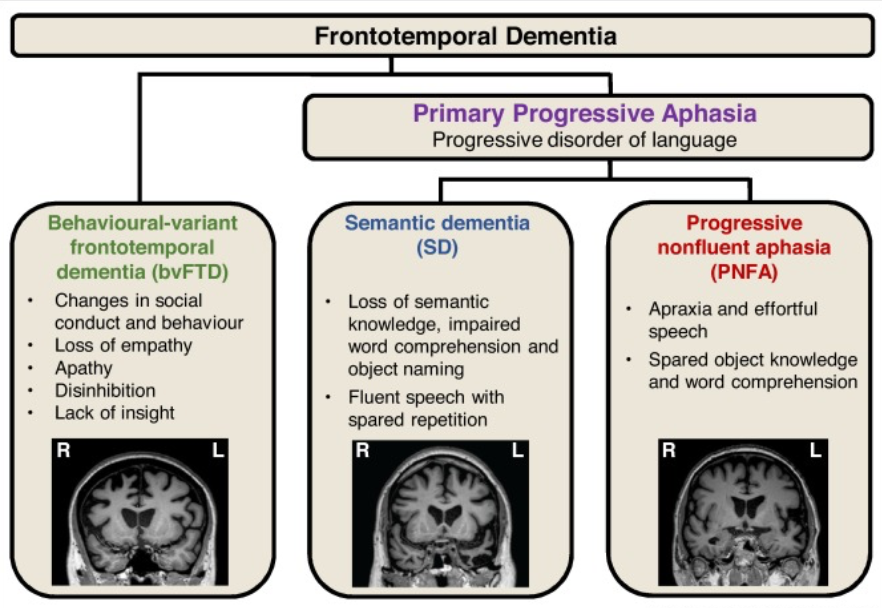
Frontotemporal dementia
No amnesia in the early stages
Clinical syndrome associated with shrinkage of the frontal and temporal lobes
Impulsive or bored and listless
Inappropriate social behaviors
Neglect of personal hygiene
Repetitive or compulsive behavior
Speech problems, semantic deficits
Types of frontotemporal dementias
Dementia with Lewy Bodies
Presence of Lewy Bodies (alpha- synuclein neuronal inclusion bodies).
Similar to AD in terms of cognitive features and can sometimes be confused with it, however it also includes other symptoms e.g.
Bradykinesia, rigidity (similar to Parkinson’s)
Recurrent and well-formed hallucinations
Memory deficits less severe than AD but visuospatial deficits are more severe than AD.
Progression of Lewy Body Dementia
Vascular Dementia
Caused by blockages in the brain’s blood supply
The second most common form of dementia (behind Alzheimer’s)
May cause or exacerbate Alzheimer’s (complicates diagnosis as vascular factors contribute to AD).
Cognitive Profile:
More impaired than AD patients on executive function
Less impaired on episodic memory
Risk factors for Vascular Dementia
High blood pressure (about 50% can be caused by hypertension)
Diabetes
High cholesterol
Family history of heart problems
Obesity
Smoking
Parkinson’s Disease
Progressive neurologic disease
Fourth most common neurodegenerative disease in the elderly
Neuropathology: degeneration of dopamine (DA) producing neurons in the brain (substantia nigra)
Motor Symptoms
Neuropsychiatric Symptoms
Major symptoms of Parkinson’s Disease
Motor Symptoms:
• Tremor
• Bradykinesia
• Rigidity
Neuropsychiatric Symptoms:
• Executive dysfunction
• Memory deficits
• Attention deficits
• Visuospatial deficits
• Mood disturbances
• Impulse control behaviors (e.g. food, drugs, gambling)
Clusters of behavioral symptoms in Alzheimer’s
Depression, apathy, sleep, agitation, psychosis
How is mild cognitive impairment linked with Alzheimer’s
12-16% progress to dementia, usually Alzheimer’s for those with memory problems
Up to 50% do not progress to dementia after 10 years
Up to 20% revert to normal cognition, though this group remains at elevated risk for dementia
How long does Alzheimer’s usually progress before the patient dies?
Average 8 years, can span from 2-20 years
What is the clock drawing test?
Normal elderly control group and dementia patients are asked to draw a clock to gauge cognitive ability. Not a diagnostic, but can help spot some symptoms of cognitive impairment
What functions of neuronal function does Alzheimer’s block?
Communication, metabolism, repair
Amyloid plaques
Present in Alzheimer’s,
Insoluble extracellular deposits which accumulate in the cortex and hippocampus.
Composed of amyloid – beta (Aß) protein fragments: Aß40 and Aß42.
Neurofibrillary tangles
Present in Alzheimer’s
Bundles of insoluble helical fibers within neurons.
Composed of hyperphosphorylated tau proteins that are normally associated with microtubules
Cortical atrophy
Loss of 1/3 of brain mass, can contribute to Alzheimers
How are amyloid and senile plaques formed?
There is normal APP in a membrane, which is broken into beta-amyloids (AB42) by an enzyme (B-secretase and y-secretase).
These beta-amyloids clump together and become amyloid plaques.
Plaques form when there is not enough clearance of AB from the brain
Genetic’s role in Alzheimer’s Disease
Point mutations in the y secretase (presenilin-1 gene) and APP gene located on chromosome 21 can lead to early onset (autosomal dominant pattern)
ApoE4 is a reliably identified genetic risk factor
Risk factors for Alzheimer’s
Age, genetics, head trauma, high blood pressure, heart disease, stroke, diabetes, high cholestrol
Individuals with Down syndrome are _________ times more likely than the general population to develop Alzheimer's disease
three to five
How does chromosome 21 influence AD?
Duplications of a small part of chromosome 21 which include the APP gene can cause early onset AD.
Point mutations in APP which cause ______________________ cause ___________________
changes in the ratio of the cleavage products, autosomal dominant early onset Alzheimer’s disease.
Protective APP mutation
A673T prevents cleavage of amyloid precursor protein (APP)
A673T protects against cognitive decline in the elderly
This finding validates a therapeutic target for Alzheimer’s disease
Molecular pathways involved in the development of Alzheimer’s
Amyloid-beta pathway
Tau protein pathology
Calcium signaling
Excitotoxicity
Changes in tau in Alzheimer’s
Tau proteins stabilize microtubules (MT) in neurons, particularly in axonal processes. MT main functions are to provide structure, organize the cytoplasm of the cell, and serve as tracks for the transport of cellular elements form the cell body to the axonal terminals (synapses).
Neuroinflammation in AD
Inflammatory cytokines, chemokines and other immune mediators are increased in tissues of individuals with AD
Prodromal Alzheimer’s Disease
Prodromal: before the development of the disease
Memory complaint corroborated by the informant
Measurable, greater-than-normal memory impairment detected with standard memory assessment tests.
Imaging the AD brain
The entorhinal cortex is first affected
Then hippocampus, amygdala and parahippocampus.Then neocortex
This loss corresponds well with NFT’s
PET Scan shows less glucose metabolism in Alzheimer’s brain
Amyloid PET Imaging for AD
Plaques can be directly imaged using amyloid tracers
TAU Pet imaging in AD
Bind to the hyperphosphorylated tau aggregates found in neurofibrillary tangles
How do CSF levels predict progression from mild cognitive impairment to Alzheimer’s?
Pathological CSF:
Aβ42: <530 ng/L
T-tau: >350 ng/L
(p-Tau)
AD Prevention
Exercise: aerobic and strength, longer than 30 minutes
Social and leisure activities
Anti-amyloid agents
Antibodies against amyloid: monoclonal antibodies can prevent clumping of amyloid-b proteins.
Agrammatism
Difficulty using grammar and syntax correctly in speech or writing
Phonemic paraphasia
Difficulties selecting the correct phoneme or sound during speech (using hat instead of bat)
TAU Function and Phosphorylation
Tau is very soluble, mostly found in astrocytes
Hyper-phosphorylated tau is insoluble and turns into tangles, leading to dysfunctional microtubule destabilization
What is a tauopathy?
A class of neurodegenerative disease that is associated with pathological aggregation of tau protein in the brain.
Primary tauopathy
Disease which have tau pathology as primary pathology, Chronic Traumatic Encephalopathy and Frontotemporal Lobar Degeneration
Secondary Tuaopathy
Disease which have Tau pathology as well as other major pathologies-eg Alzheimer disease is a secondary tauopathy, it is also an amyloidosis-aggregation of senile plaques
Chronic Traumatic Encephalopathy
Progressive degenerative disease occurring with those with multiple concussions, common in athletes or war veterans
Pathological features of CTE
Tau deposition as neurofibrillary tangles
Changes in white matter
Glial degeneration
Beta-amyloid deposition is uncommon
Frontotemporal degeneration
Three subtypes
Progressive nonfluent aphasia (affects left frontal lobe 1st)
Semantic dementia (affects left anterior temporal lobe 1st)
Behavioral variant frontotemporal dementia (affects right frontal lobe 1st)
All share some common features
Progressive decline in frontal and temporal lobes
Progressive nonfluent aphasia
Starts focally in the left side of the frontal lobe
Broca’s area primarily affected - speech production
As the disease develops, speech quantity decreases and many patients become mute.
Semantic dementia
A number of frontotemporal lobar degeneration cases start in the anterior temporal lobes (left more than right)
Loss of semantic knowledge (loss of word meanings)
Starts with word-finding problems
In a subgroup of patients there is a dramatic increase in visual creativity
Behavioral Variant FTD
Begins in the frontal lobes
Orbitofrontal cortex: Involved in emotional processing
Right sided damage first - social behavioral impairments
Loss of the anterior cingulate which is critical for motivational behaviors and conflict resolution
Genetics of FTD
Approximately 40% of people with FTD have a family history of FTD or another related dementia
5-10% of patients have autosomal dominant inheritence pattern
Prion’s disease are also known as…
transmissible spongiform encephalopathies (TSEs)
What is Prion Disease/TSE
A family of rare progressive neurodegenerative disorders that affect both humans and animals.
Distinguishing features of Prion’s diseases
Long incubation period, spongiform changes associated with neuronal loss, failure to induce inflammatory responses.
Caused by prions (a type of protein)
What are prions?
Proteinaceous infectious particles refers to abnormal proteins that can induce misfolding of normal cellular proteins.
PrPC is the __________ form of the prion protein, while PrPSc is the form ___________.
normal, cellular
abnormal, misfolded, and infectious
How do prions multiply?
By a nucleation and fragmentation process similar to the growth of crystals.
Mad cow disease
Prion’s disease in cows. Researchers found that if you inject protein denaturant to inactive proteins (bleach) then you will not get the disease, showed that the disease is caused by protein malfunctions.
Normal function of the prion protein
Located in pre and postsynaptic sites
Regulate NT receptor function and maintain myelin sheath
Play a role in memory, long-term potentiation and sleep
Structural changes in prion’s proteins
More pleated sheets than alpha helices in an abnormal protein
Abnormal prion protein starts interacting with normal ones and makes it pathological, kills the cell, then released from that one and propagates
Glial cells proliferate, neurons die and the brain appears spongy, which is why they are called spongiform encephalopathies
How is prion’s spread?
From eating infected brains,
Human TSE types
Creutzfeldt-Jakob disease
Kuru
Fatal Familial Insomnia
TSE subtyping
Genetic
Sporadic
Acquired
Genetic TSE subtype
Caused by a mutation in the PrPc protein (10-15% of all TSE)
Genetic Creutzfeldt–Jakob disease (gCJD)
Fatal familial insomnia (FFI)
Gerstmann–Straeussler–Scheinker syndrome (GSS)
Sporadic TSE subtype
80-95% of all TSEs
Sporadic CJD
Sporadic FFI
Acquired TSE subtype
Variant CJD
Kuru
Creutzfeldt-Jakob Disease (CJD)
Very rare, 1 in a million
Can be sproadic, acquired, familial
Very rapid progression and deterioration of cognitive function