Molecular Geometry
1/19
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
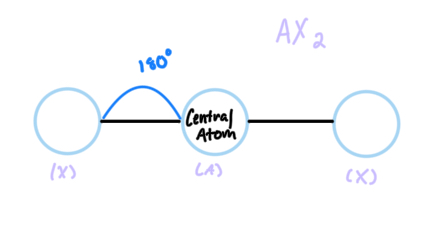
Linear
AX2, sp hybridized
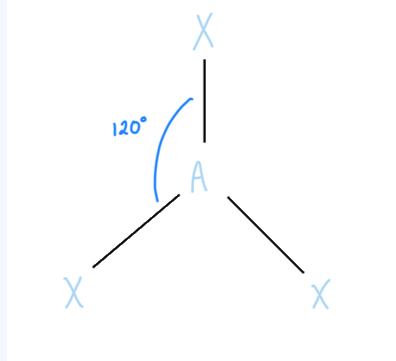
Trigonal Planar
AX3, sp2 hybridized
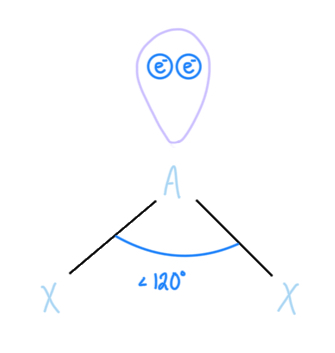
Bent
AX2E, sp2 hybridized
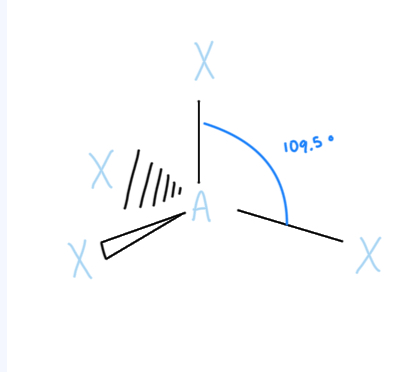
Tetrahedral
AX4, sp3 hybridized
Trigonal Pyramidal
AX3E, sp3 hybridized
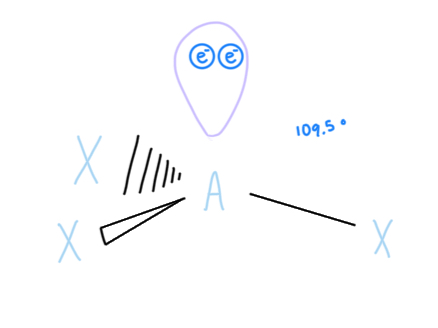
Bent (w/ 2 lone pairs)
AX2E2, sp3 hybridized
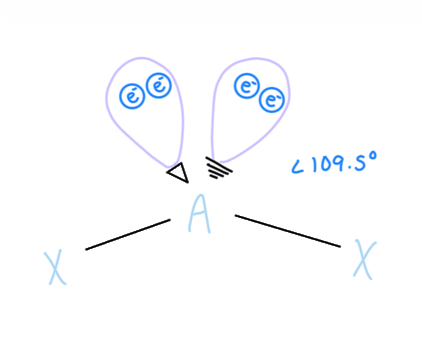
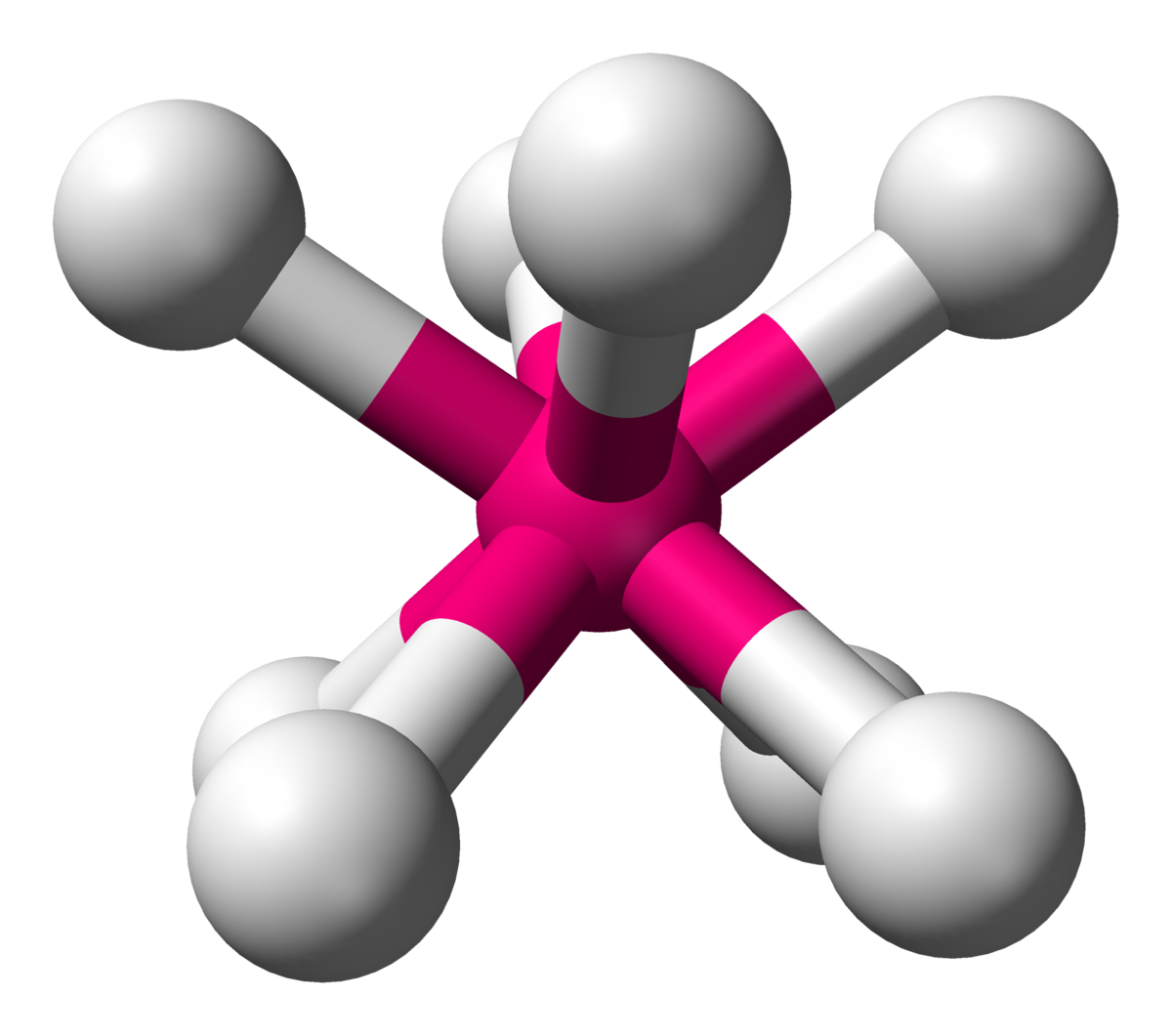
Trigonal Bipyramidal
AX5, sp3d hybridized
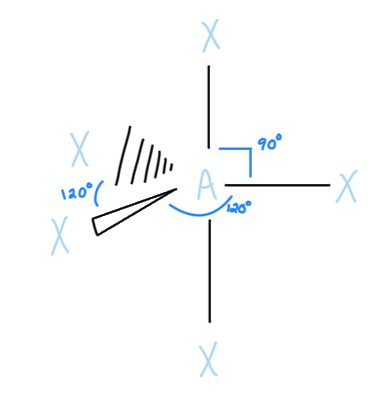
Seesaw
AX4E, sp3d hybridized
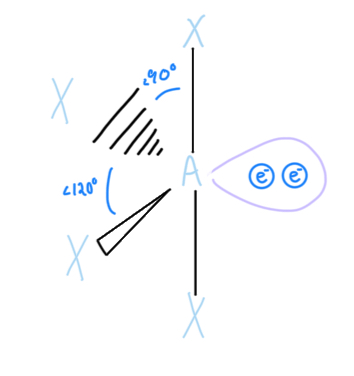
T-shaped
AX3E2, sp3d hybridized
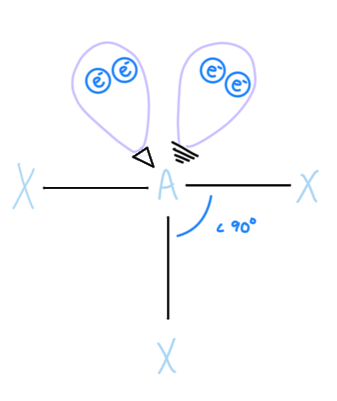
Linear (w/ 3 lone pairs)
AX2E3, sp3d hybridized
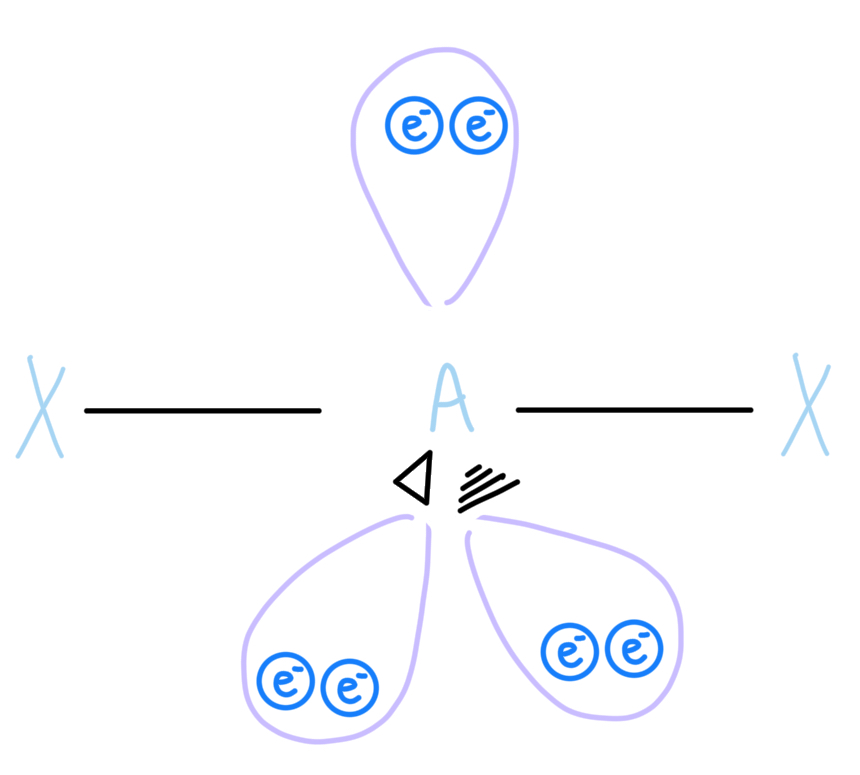
Octahedral
AX6, sp3d2 hybridized
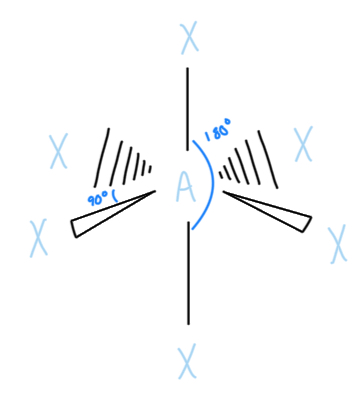
Square Pyramidal
AX5E, sp3d2 hybridized
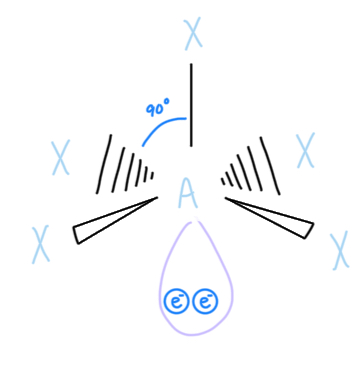
Square Planar
AX4E2, sp3d2 hyrbidized
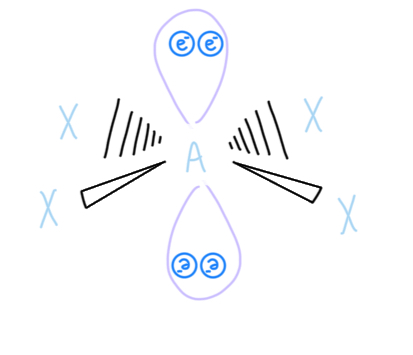
Pentagonal Pyramidal
AX6E1, sp3d3 hybridized
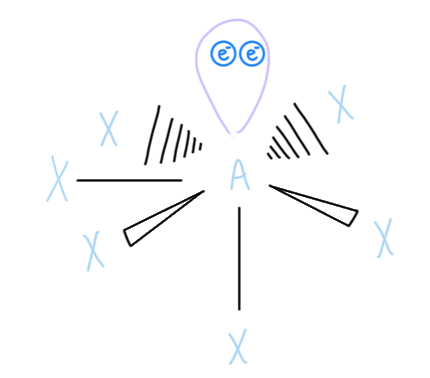
Pentagonal Bipyramidal
AX7, sp3d3 hybridized
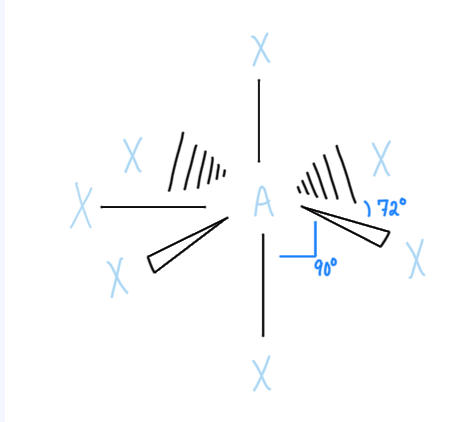
Pentagonal Planar
AX5E2, sp3d3 hybridized
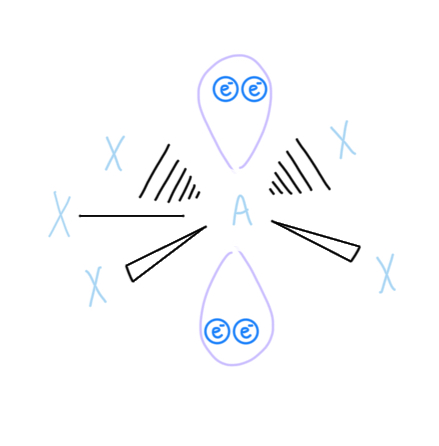
Square Antiprismatic
AX8, sp3d4 hybridizded
Steric number
Number of bonds on a central atoms (bonds + lone pairs)
Hybridization
Combining two atomic orbitals to create hybridized orbitals
Dipole moment
Measurement of the polarity of a molecule (containing two opposite electrical charges)