Topic 5 - Separate Chemistry I ✅
1/41
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Describe transition metals
• Most metals are transition metals
• Their properties are:
○ High melting point
○ High density
○ Form coloured compounds
○ Can be used as catalysts
What does the oxidation of metals result in
• Corrosion
• E.g. Rusting
How can rusting be prevented
• Exclusion of oxygen or water
• Sacrificial protection
Explain how rusting can be prevented by exclusion of water
Storing the metal with a desiccant powder, as this absorbs water vapour.
Explain how rusting can be prevented by sacrificial protection
• A piece of magnesium/zinc is attached to the iron/steel object.
• Magnesium/zinc oxidize more easily than iron, so they react with oxygen instead of the iron/steel.
• This protection continues until the sacrificial metal corrodes away.
What is electroplating
electroplating uses electrolysis to put a thin layer of a metal onto a metal object
Explain how electroplating can be used to improve the appearance and/or the resistance to corrosion of metal objects
• To improve appearance, expensive metals such as silver and gold can be used.
• To improve a metal's ability to resist corrosion, chromium is often used because it stops air and water reaching the steel below, preventing it from rusting
Why are alloys stronger than pure metals
• In alloys there are atoms of different sizes.
• This disrupts the regular structure of the atom
• This means that a greater force is required for the layers to slide over each other.
• Therefore alloy is harder and stronger than the pure metal.
Explain why iron is alloyed with other metals to produce alloy steels
• Pure iron is too soft for everyday use, but alloy steels are strong
• Alloys can be designed for specific uses
Uses of steels
• Low carbon steels - used for sheeting because they are malleable
• High carbon steels - used for cutting tools because they are hard
• Stainless steels - used for cutlery because they are resistant to corrosion
Use of aluminium
• Aircraft
• Because it is low density
Use of copper
• Electrical cables
• Because it is a good conductor
Use of gold
• Jewellery
• Because it has good resistance to corrosion
Use of magnalium
• Cars and planes
• Because it is low density
Use of brass
• Coins
• Because it is hard and resistant to corrosion
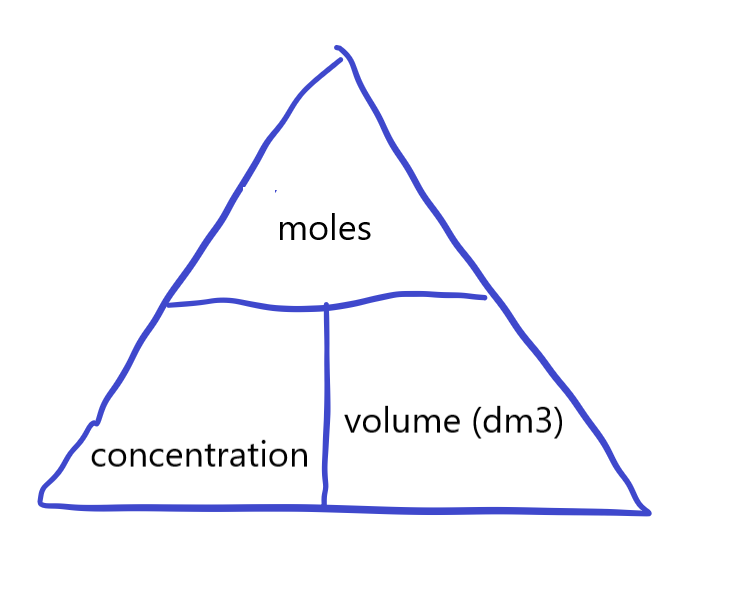
Calculate concentration of solutions in mol dm-3
• Moles = conc x vol
• Divide by 1000 to convert cm3 to dm
Method for carrying out an acid-alkali titration
• Add acid to burette using a funnel, record the volume in the burette to start
• Add known volume of alkali to a conical flask and add some indicator
• Place conical flask on white tile (so you can see colour change clearly)
• Add acid to alkali until you reach the end point
• Calculate how much acid has been added (titre
• Repeat until you get concordant titres
ESQ - Describe how to carry out a titration to find the exact volume of sulfuric acid needed to neutralise 25cm of sodium hydroxide solution
• Rinse pipette with alkali and burette with acid
• Measure alkali using a pipette into a beaker and place beaker on white tile
• Add phenolphthalein indicator
• Fill burette with acid and read volume of acid
• Add acid to the beaker, slowly swirling until phenolphthalein goes colourless
• Read volume of acid in burette at end of titration
• Repeat until concordant results
How to calculate the concentration of the alkali if you're given the concentration of the acid (for titration questions)
• Calculate moles of acid
• Concentration x volume = moles
• Calculate the mole ratio of acid to alkali
• Work out how many moles of alkali you have using the mole ration and moles of acid
• Calculate the concentration of the alkali
• Concentration = mol / volume
Formula for percentage yield
• (Amount of product produced / maximum amount of product possible) x 100
• This is the same as Actual / theoretical x 100
Why is actual yield less than theoretical yield
• Incomplete reactions
• Practical losses during the reaction
• Unwanted side reactions
what is atom economy
A measure of the amount of starting materials that end up as a useful product
formula for atom economy
(Mr of desired product / sum of Mr of all reactants) x 100
Explain why a particular reaction pathway is chosen to produce a specified product
If the pathway has __ it is more likely to be chosen:
• high atom economy
• high yield
• fast rate of reaction
• useful by-products
How to calculate the molar volume of any gas at room temperature
• Volume (dm3) of gas at room temp = moles x 24
• Volume (cm3) of gas at room temp = moles x 24000
Describe what molar volume is
Molar volume of any gas at room temperature is the volume occupied by one mole of molecules at room temperature
How to calculate mass of a product when given the mass of the reactant
• Calculate moles of the reactant
• if is a mass do mass / molar mass
• If it is a volume do volume / 24
• Work out the mole ratio
• Calculate mass / volume using moles
• For calculating mass do moles x molar mass
• Or calculating volume do moles x 24
How to calculate the number of particles in 1 mole
One mole contains 6.02 x 1023 particles
Describe the Haber process
• A reversible reaction
• Between nitrogen and hydrogen
• To form ammonia
How does the rate of attainment of equilibrium change when the temperature changes
• When a higher temperature is used, the equilibrium will be reached at a faster rate
• This is because particles have more kinetic energy -> collide more frequently -> have more successful collisions
How does the rate of attainment of equilibrium change when the pressure changes
• The equilibrium is reached at a faster rate when a higher pressure is used
• More particles in a given volume -> more frequent, successful collisions
How does the rate of attainment of equilibrium change when the concentration changes
• The equilibrium is reached at a faster rate when a higher concentration is used
• More particles in a given volume -> more frequent, successful collisions
How does the rate of attainment of equilibrium change when a catalyst is used
Equilibrium is reached at a faster rate when you use a catalyst
Explain how the Haber process' conditions are related to the availability and cost of raw materials and energy supplies
• Catalysts help increase rate of reaction but they are expensive
• High temperatures and pressures can be expensive and dangerous
• So the equipment needed for them can be very expensive
Explain how the Haber process' conditions are related to the control of temperature, pressure and catalyst used to produce an acceptable yield in an acceptable time
• High temperatures and pressures increase rate of reaction
• But a higher temp shifts equilibrium towards the reactants
• Therefore a compromise is required to ensure a fast rate of react and a high yield of products
What do fertilisers contain and why
• Nitrogen, phosphorus and potassium compounds
• To promote plant growth
Describe how ammonia reacts with nitric acid
• Ammonia acts as a base
• Ammonia + nitric acid -> ammonium nitrate
• The product of this reaction is a salt that can be used as a fertiliser
Describe how ammonium sulfate can be prepared in the lab
• Reactants - ammonia solution and dilute sulfuric acid
• Produces a little bit of ammonium sulfate
• Involves titration then crystallisation
Describe how ammonium sulfate can be prepared industrially
• Reactants - natural gas, air, water (to make ammonia). Sulfur, air, water (to make sulfuric acid)
• Produces a lot of ammonium sulfate
• Many stages required
What do chemical cells do
They produce a voltage until one of the reactants has been used up
Describe a hydrogen-oxygen fuel cell
Hydrogen and oxygen are used to produce a voltage
Water is the only product
Pros and cons of fuel cells
Pros
Produce only water as waste
Keep producing fuel if fuel keeps being supplied
Cons
Difficult to transport and store, therefore not suitable for portable devices
Expensive to make