electrochemistry
1/142
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
143 Terms
displacement reaction
When one element takes the place of another we call it a displacement reaction. In this reaction zinc displaces copper because zinc is a stronger reducing agent than copper.
"What is a displacement reaction, and what does it tell us about reactivity?"
When one element takes the place of another element we call it a displacement reaction. ZInc displaces copper ions from copper sulfate solution. But copper does not displace zinc ions from zinc sulfate solution and this tells use zinc is a stronger reducing agent than copper
"How does the electrochemical series rank reducing power?"
"Lazy Kangaroos Can Make Zebras Feel Nice, Playing Cool Classical Funky Anthems."
The electrochemical series ranks the reducing power of half equations. Reading each half equation forward shows the ion acting as an oxidizing agent and reading each equation backwards gives us the metal acting as a reducing agent.
Could this reaction take place ?
"Why does iron commonly form Fe²⁺ rather than Fe³⁺ in displacement reactions?"
Iron can exist as Fe²⁺ or Fe³⁺ in solution.
Fe²⁺ (oxidation state +2) is the more common oxidation state in displacement reactions like this one.
Fe³⁺ (oxidation state +3) is less likely to form directly from metallic iron in this context because forming Fe³⁺ requires more energy (a higher oxidation potential).
The reducing ability of a substance depends on its tendency to lose electrons.
Fe (iron metal) is a good reducing agent because it easily loses electrons to form Fe²⁺. However, Fe³⁺ is not directly relevant here because Fe would first oxidize to Fe²⁺ during the reaction.
predicting the direction of redox reactions
The electrochemical series allows us to predict the direction of redox reactions because stronger reducing agents cannot be reduced by weaker reducing agents. They can only be oxidized.
Examples of how the electrochemical series be used to predict displacement reactions?"
For example Calciums row is above zinc's row. The electrochemical aries tells us that calcium is a stronger reducing agent than nickel so we can predict that if we add calcium to a solution containing nickel ions a displacement reaction will take place where calcium reduces nickel ions and nickel ions oxidise calcium
We can also predict that if we add nickel to a solution of calcium ions no reaction will take place. Nickel cant reduce calcium ions because nickel is a weaker reducing agent that calcium
"How does the gap between oxidizing and reducing agents affect the energy released in a reaction?"
The bigger the gap between oxidizing and reducing agents the more the energy the reaction will give out. For example the gap between zinc and magnesium is small so when magnesium reduces zinc ions the reaction is not very vigorous and not much energy is given out. But when magnesium reduces copper ions the reaction gives out so much energy that it is explosive. All this energy comes from the movement of two electrons on magnesium to copper
"How can moving electrons be used to generate useful energy?"
If we can get the electrons to move through a wire we can harness their power and make them do something useful on the way like power a lightbulb
creating a half cell: step 1
We can do this by separating the half equations:
creating a half cell: step 2
Copper ions are reduced from copper sulfate to solid copper. There's also a piece of solid copper dipped into the collusion so that the copper ions which are reduced will bond to its surface rather than falling onto the floor
creating a half cell: step 3
Magnesium will be oxidized to magnesium ions. We start with magnesium and magnesium sulfate (just like for copper)
creating a half cell: step 4
This means we have two beakers each containing a metal and solution of that metal ions.
A metal in a solution of its own ions is called a half cell,
definition of a half cell
define
half cell
cell
battery
"Do reactions occur in half-cells before they are connected?"
Even though we haven't connected the two half cells together reactions are already happening inside the two beakers
"What happens at the molecular level during a slow redox reaction?"
This is a slow reaction. Here and there an ion picks up two delocalised electrons and forms a copper atom. The reverse is also happening as here and their metallically bonded copper ions drift into the solution as copper ions
"What is a reversible reaction, and when does it reach dynamic equilibrium?"
A reaction which proceeds in both directions is called a reversible reaction and in a close system a reversible reaction will eventually reach dynamic equilibrium (rate of forward reaction = rate of backward reaction)
"Why is magnesium a stronger reducing agent than copper?"
An equilibrium is also happening in the other half cell with some magnesium ions going into the solution and some coming out of the solution to become part of the strip of metal. However magnesium forms ions in the solution more readily than copper does which explains why magnesium is a stronger reducing agent.
This means there are more magnesium ions in the solution than copper ions and their is a higher concentration of delocalised electrons in the strip of magnesium than in the strip of copper.
"How does the position of the equilibrium affect the concentration of ions and electrons in magnesium and copper reactions?"
There are more magnesium ions in the solution than copper ions and their is a higher concentration of delocalised electrons in the strip of magnesium than in the strip of copper.
In other words the position of the magnesium equilibrium is to the left of the position of the copper equilibrium. Because with the magnesium reaction the concentration of the reactants is greater than is in copper reaction.
"How does the position of the equilibrium affect the concentration of ions and electrons in magnesium and copper reactions?"
"Why do electrons flow from the magnesium strip to the copper strip in a galvanic cell?"
For stronger reducing agents the equilibrium lies further to the left. This means there is a higher concentration of delocalised electrons in the reducing agent (stronger one - in this case magnesium) than copper. This means that the charge in the stirp of magnesium is more negative. So if we connect the electrodes with a wire the electron will flow from the area of negative charge to the area of positive charge. This flow is electrical current.
"What happens to the equilibrium in the copper half-cell when electrons flow from the magnesium strip?"
As more electrons flow from the magnesium to the copper the equilibrium in the copper half cell is disturbed because the concentration of one of the reactants has increased (2e-). This means the of the equilibrium shifts to the right. This means that more copper ions come out of the solutions and become metallically bonded copper atoms balancing out some of the new delocalised electrons position
"What happens to the equilibrium in the magnesium half-cell as electrons flow, and how does it affect the flow of electrons?"
In the magnesium half cell (reducing agent) the decreasing concentration of reactants causes the equilibrium to shift to the left meaning more magnesium atoms become ions in the solution and with fewer cations more delocalised electrons are free to flow through the wire
"How does the flow of electrons from the magnesium electrode affect the magnesium half-cell equilibrium?"
For understanding purposes
As the electrons are removed from the magnesium electrode and flow through the wire, the local balance of charge is disrupted.
This makes the equilibrium shift to the left to replace the lost electrons, which involves the oxidation of magnesium atoms.
Initially, more Mg2+ ions are produced because magnesium atoms oxidize to release electrons.
However, the removal of electrons (via the wire) occurs faster than Mg2+ ions can disperse into the surrounding solution
With fewer Mg2+ ions locally, there is less positive charge near the electrode.
The lack of positive charge (cations) means the delocalized electrons in the magnesium metal experience less attraction to stay in place.
As a result, the electrons can flow more freely through the wire toward the copper half-cell.
"What happens when two half-cells are connected in an electrochemical cell?"
When the two half cells are connected electrons flow from the reducing agent (more negatively charged) through the wire to the less negatively charged metal. As the concentration of reactant changes the equilibrium in each half cell is disturbed. For the reducing agent the position of the equilibrium shifts to the left sending more electrons through the wire. For the oxidizing agent the position of the equilibrium shifts to the right and more ions come out of the solution balancing out some of the new electrons.
LEARN THIS SUMMARY
"What does the potential difference between two electrodes reflect, and what factors can affect it?"
The actual voltage measured between two electrodes is the potential difference. The potential difference reflect the difference in reducing strength between the two electrodes(ie. magnesium and copper). The potential difference is affected by factors like the resistance in wires. If we use wired with less resistance we can get slightly higher voltage.
"What is electromotive force (E), and how does it relate to the voltage between two electrodes?"
The electromotive force (E) is the maximum possible voltage between two electrodes. It ISN’T affected by factors like resistance. The electromotive force reflect the difference in reducing strength between the two electrodes
At A level you tend to treat potential difference and electromotive force as the same.
"Why is the hydrogen electrode used as a reference in measuring reducing power?"
We can only give the value of the reducing power of one electrode by comparing it to another electrode (calculating the EMF between them). However there are loads of pairs of electrodes and we can't give values for every possible pair so chemists pick hydrogen and we compare everything else to hydrogen. This is because its cheap, readily available and around the middle of the list (of the electrochemical series.
How do we make a hydrogen electrode when hydrogen is not a metal
We connect a wire to a strip of platinum which will conduct electricity but won’t react with anything and dip it in a solution containing H+ like HCl (1 mol/dm3) acidor sulfuric acid (0.5 mol). Then bubble hydrogen gas through the solution.
"What happens at the hydrogen electrode when the other electrode is a stronger reducing agent?"
If the other electrode is stronger reducing agent than hydrogen: hydrogen ion (H+) picks up electrons form the platinum and are reduced to hydrogen gas (as the electrons will move to from the stronger reducing agent to hydrogen)
"What happens at the hydrogen electrode when the other electrode is a weaker reducing agent?"
If the other electrode is a weaker reducing agent than hydrogen: The hydrogen gas is oxidised to the hydrogen ions (H+) and the electrons (2e-) flow through the platinum strip to the other electrode.
"How is electrode potential measured, and what does it indicate about the strength of reducing and oxidizing agents?"
Each electrode can be given an electrode potential which is always measured in volts and always relative to hydrogen. Stronger reducing agents than hydrogen have a negative electrode potential and stronger oxidizing agents than hydrogen have a positive electrode potential.
Work out magnesium electrode potential
Magnesium is a stronger reducing agent than hydrogen (higher than hydrogen in the table), This means that magnesium will be the negative electrode. This means magnesium electrode potential is -2.37. (REMEMBER TO INCLUDE THE NEGATIVE SIGN)
Work out coppers electrode potential
Hydrogen is a stronger reducing agent than copper (higher on the table). This means hydrogen is the negative electrode and copper is the positive electrode. So copper's electrode potential is positive (0.34+). MAKE SURE TO ADD THE + SIGN. DON’T ADD - SIGN TO HYDROGEN ELECTRODE 0.00V is the standard for hydrogen everywhere.
"What are standard electrode potentials and under what conditions are they measured?"
These electrode potentials are what we observe under standard conditions so they are referred to as standard electrode potentials (298K, 100kPa, 1 mol/dm^3). They tell the electromotor force between each electrode and hydrogen electrode at standard temperature, pressure and concentration
what are standard conditions
Standard conditions
Temperature = 298 Kelvin
Pressure = 100 kPa
Concentration = 1mol dm^-3
how do we represent a standard electrode potential
To represent a standard electrode potential we use (Delta E)
"How does Le Ch atelier's Principle apply to changes in non-standard conditions in electrochemical cells?"
We don’t use the standard electrodes potential sign here because the acid wasn’t at 1mol/dm^-3)
Using Le Ch atelier's Principle, we can predict how changes in non-standard conditions (e.g., concentration, pressure, or temperature) affect the electrode potential in an electrochemical cell.
Applying Le Chatelier's Principle to non standard concentration.
What happens to the electrode potential of a hydrogen electrode if we lower the pressure of hydrogen gas
This is in non standard conditions as the zinc concentration is less than 1 mol/dm^-3. Lowering the concentration of the solution means lowering the concentration of zinc ions. Zinc ions are one of the reactants and if we have a lower concentration of the reactant the equilibrium moves to the left which means the reaction produces more electrons. This makes the charge of the zinc strip more negative and so the electron potential becomes more negative (-0.76V to -0.93V)
Lower pressure: The system shifts to produce more gas particles (H2)
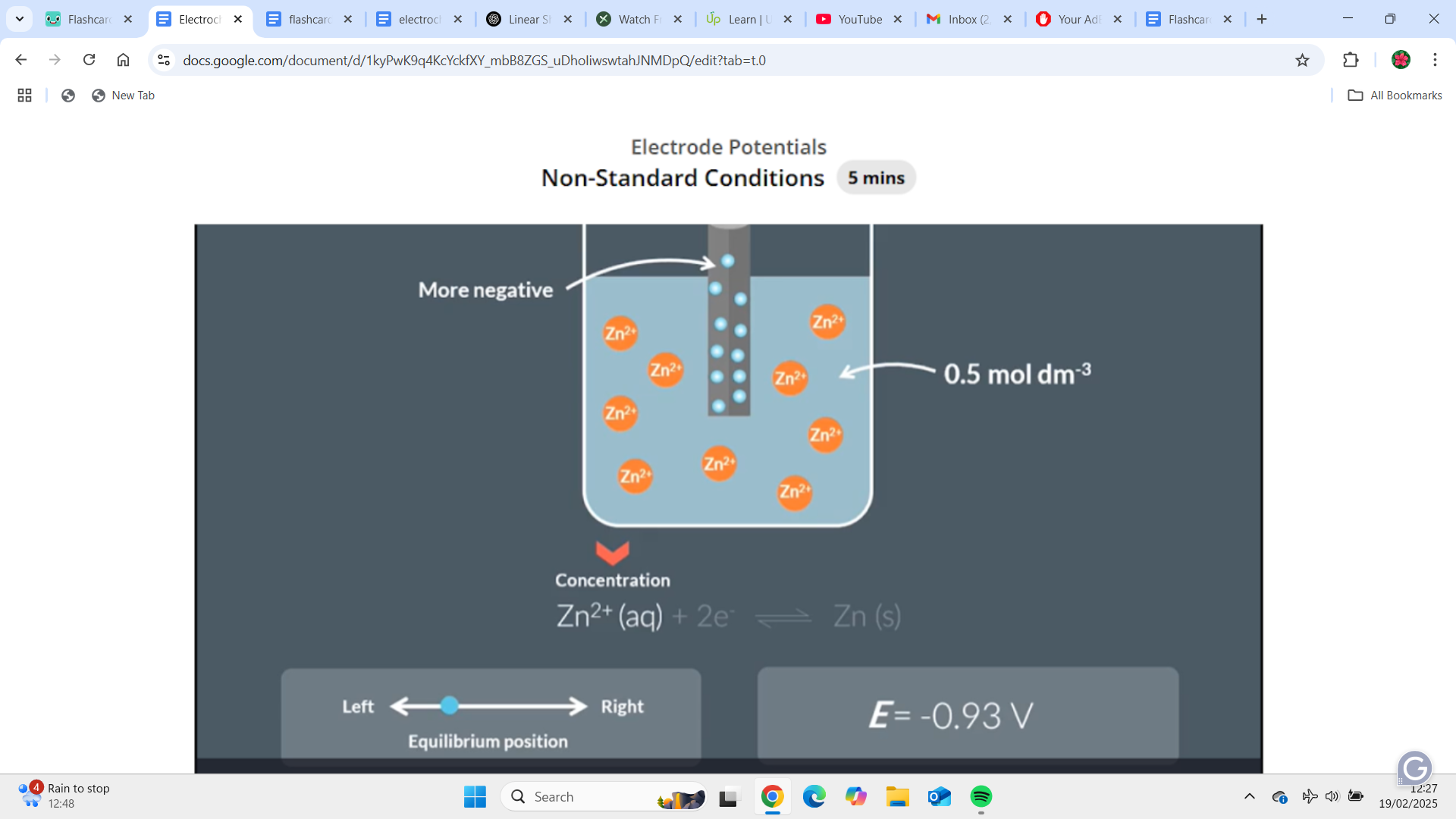
"How does Le Chatelier's Principle apply to changes in non-standard conditions in electrochemical cells?"
We can work out the effect of non standard by thinking about the position of equilibrium. If the position of equilibrium shifts to the left then more electrons are produced and the electrode potential becomes more negative
"What happens to the electrode potential if the position of equilibrium shifts to the right?"
IF the position of equilibrium shifts to the right than fewer electrons are produced and the electrode potential becomes more positive
"How is electromotive force (EMF) conventionally reported in electrochemical cells?"
When we give an electromotor force we give the polarity of the right hand electrode and its convention to put the positive electrode on the right so usually the sign of the electromotor force is positive and we only get a negative sign if the negative electrode is on the right (if the reducing agent is on the right).
We read left to right. Just like how we arrange cells so that the electrons move left to right and we give the voltage of the cell going left to right. (IUPACS Convention)
"What is the difference between a negative electromotive force (EMF) and a negative electrode potential?"
A negative electron motor force means that a reducing agent is on the right but a negative electrode potential just means the electrode is a stronger reducing agent than hydrogen. So International Union of Pure and Applied Chemistry (IUPAC) only applies to the electromotive force for a cell AND NOT to standard electrode potentials
"How is the electromotive force (EMF) of a cell calculated, and what does a negative value indicate?"
Cells EMF = right hand electrode potential - left hand electrode potential. IF the value it produces is negative than the negative electrode is on the right but by convention its normally on the left.
"How can you use electrode potentials to check if your EMF calculation is reasonable?"
Sketch this digram to check your answer. Lithium electron potential tells us the gap between lithium and hydrogen. We also know the gap between copper and hydrogen. Draw a line going from lithium to copper and use this line to check your answer is reasonable.
"How can you use electrode potentials to check if your EMF calculation is reasonable?"
You can also use the sketch to check the sign of the EMF>If the electrode arrangement on the diagram given does not match the sketch then the sign of the cell's EMF is negative. E.g in the diagram zincs electrode is on the left butt on the sketch its on the right this means the sign of the cells EMF is negative.
"Why does the voltage drop to zero when two half-cells are connected by a wire?"
When two half cells connected by a wire positive charge builds up in the left hand solution and negative charge builds up in the right hand solution. Both of which bring thise reaction to a halt which explain why the voltage drops to 0.
Explain why the voltage drops
When two half cells are connected electrons flow away from the magnesium electrode towards the copper electrode. The more electrons that leave the strip of magnesium the more magnesium cations are able to go into the solution. At first the more electrons that leave the strip of magnesium the more magnesium cations (MG2+) are able to go into the solution. But as more magnesium captions go into the solution the charge of the solution becomes more and more positive. This causes cations to stop going into the solution and electrons are no longer free to flow into the wire. So the voltage drops
Explain why the voltage drops
In the copper half cell at first more electrons in the wire flow into the strip of copper and more copper cations come out of the solution and bond with the electrons to the calcium strip. But as copper cations (Cu2+) come out of the solution the solution charge becomes more and more negative. This stops copper cations from coming out of the solution and bonding with the calcium strip. Without the cations balancing out the incoming electrons the negative charge builds up in the strip of the copper. So the voltage drops.
"What is the purpose of a salt bridge in an electrochemical cell?"
A salt bridge prevents the buildup of charge by allowing the movement of ions between the two solutions. Cations move into the collusion which is losing cations and anions move into the solutions which are gaining cations. We call it a salt bridge because it contains cations and anions which together make a salt. A salt bridge completed an electrical circuit allowing our cell to keep generating a constant voltage until there are no more ions in the salt bridge/magnesium dissolved/all copper ions have come out of the solution.
"How does a salt bridge prevent charge build up in an electrochemical cell?"
We prevent the build up of charge by adding cations and anions. If we soak a tissue in a solution of anions and cations so that the anions (- charge) will be drawn to the solution that becomes positively charged. The cations (+) will be drawn to the solution that becomes negatively charged. Or we can use a glass tube with cotton wool at either end so that the ions diffuse gradually. We call any arrangement like this a salt bridge.
"Why is potassium nitrate (KNO₃) commonly used in a salt bridge?"
When choosing a salt its important to choose ions that won't react with ions in the solution as this would alter the electromotive force of the cell. Usually we use potassium nitrate (KNO3) because it does not react with ions in the solution of most electrodes.
In the exam you may be asked why a certain shouldnt be used with a certain electrode but it will only ask you abbott reactions that you learn elsewhere in the course. E.g in the halides topic you learn hwy chloride salt hsouldnt be used with a slievr electrode
This changes the concentration of hydrogen (less than 1 mol dm-3) in the solution which alters the electromotive force between the two electrodes. Similarly if carbonate ions ended up in metal electrode then the metal carbonate would precipitate and this would change the concentrations (less than 1 mol dm-3) and alter the electromotive force
the conventional representation of cells
Look at the value of the Ecell. Cell diagram should be drawn negative to positve
IDentift states (aq to aq or aq requires Pt)
Write Pt and Metal electrode at the start end with sijngle line
Split the cations with a double line
Two aq should be seperated with a comma
Example of a cell diagram
"What three states of matter are present in a hydrogen electrode, and which component is placed next to the salt bridge?"
In hydrogen electrode there are components in three states. Solid platinum, aqueous H+ ions and hydrogen gas. The H+ are the most oxidized substance so they go next to the salt bridge.
If there is more than one substance in the same state in the same container we separate them with commas.
In this cell there are two aqueous substances in the same container so we separate them with commas and put the more oxidised one next to the salt bridge
The IUPAC conventions for the representation of electrochemical cells cover 6 points. These are:
Take away the right side of the equation form the left
More negative value goes left. More positive value goes right
correct cell representation
Same state use comma
Fe3+ higher oxidation than Fe2+
We need to add Pt(s) because Fe3+ and Fe2+ exist as aqueous solutions and we need a solid conductor.
7 specific cells
"How is the Daniel cell similar to a battery, and how does the setup differ from an AA battery?"
The daniel cell consists of a zinc half cell connected to a copper half cell which is similar to a battery. Th negative electrode to a AA battery is zinc but rather then being in a separate container the zinc forms the outside of the battery and the positive electrode is on the inside.
"Why is a separator used in an AA battery, and what is its role?"
In an AA battery, the reducing and oxidising agents are close together. A separator is used to keep them from reacting directly. However the separator is POROUS which allows the transfer of IONS. This is necessary to PREVENT THE BUILD UP OF CHARGE AROUND EACH ELECTRODE.
what are dry cells
Modern batteries are often dry cells. Dry cells contain no free LIQUID. Instead they contain PASTE which still allows IONS to move
"What is an electrolyte, and how does it help maintain the function of a battery?"
A substance which contains ions that are free to move thereby completing an electrical circuit is called an electrolyte. Any substance which forms cations and anions in a solution is called an electrolyte. And these anions and cations move across a separator or salt bridge to prevent the buildup of charge and keep the battery running.
"In a Daniel cell, what are the electrolytes and the salt bridge
In a daniel cell the zinc sulfate and copper sulfate are electrolytes and the potassium nitrate in the salt bridge is also an electrolyte
"In a dry cell (AA battery), how does the electrolyte allow ions to move despite the absence of free liquid?"
In a AA battery the electrolyte is the paste next to the zinc in which the ions can still move since a dry cell contains no free liquid but still contains enough liquid for the ions in the electrolyte to move
Not a priority to learn but familiarising youself with these batteries will be useful in the exams
"What is the difference between the electrolyte in a zinc-carbon battery and a zinc-chloride battery?"
Instead of zinc chloride paste a zinc-carbon (Standard AA battery) cell contains ammonia chloride paste