Unsaturated Hydrocarbons + Alkenes/Alkynes/Alkyl Halides
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
what are unsaturated HC's + why this happens
= have less than the max amnt of H's bcs instead of only single bonds, they have 2 or even 3 bonds b/w the C's > bcs dble and triple bonds are there, the carbons have less bonds available with the H's
name for molecules w. double bonds vs triple
double bonds - alkenes
triple bonds - alkynes
general eqn to get the formula of an alkane vs alkene vs alkyne vs cycloalkanes + how to rmember if forgettign
alkanes: CₙH₂ₙ₊₂
alkenes: CₙH₂ₙ
alkynes: CₙH₂ₙ-₂
***every additional bond b/w Carbons removes 2 H's from molecules, one from each Carbon making the extra bond
cycloalkanes: CₙH₂ₙ
^^rings have same formulas as alkenes bcs each C is already attached to 2 C beside it so each C can only bond with 2H's out of its 4 bond possiibilities
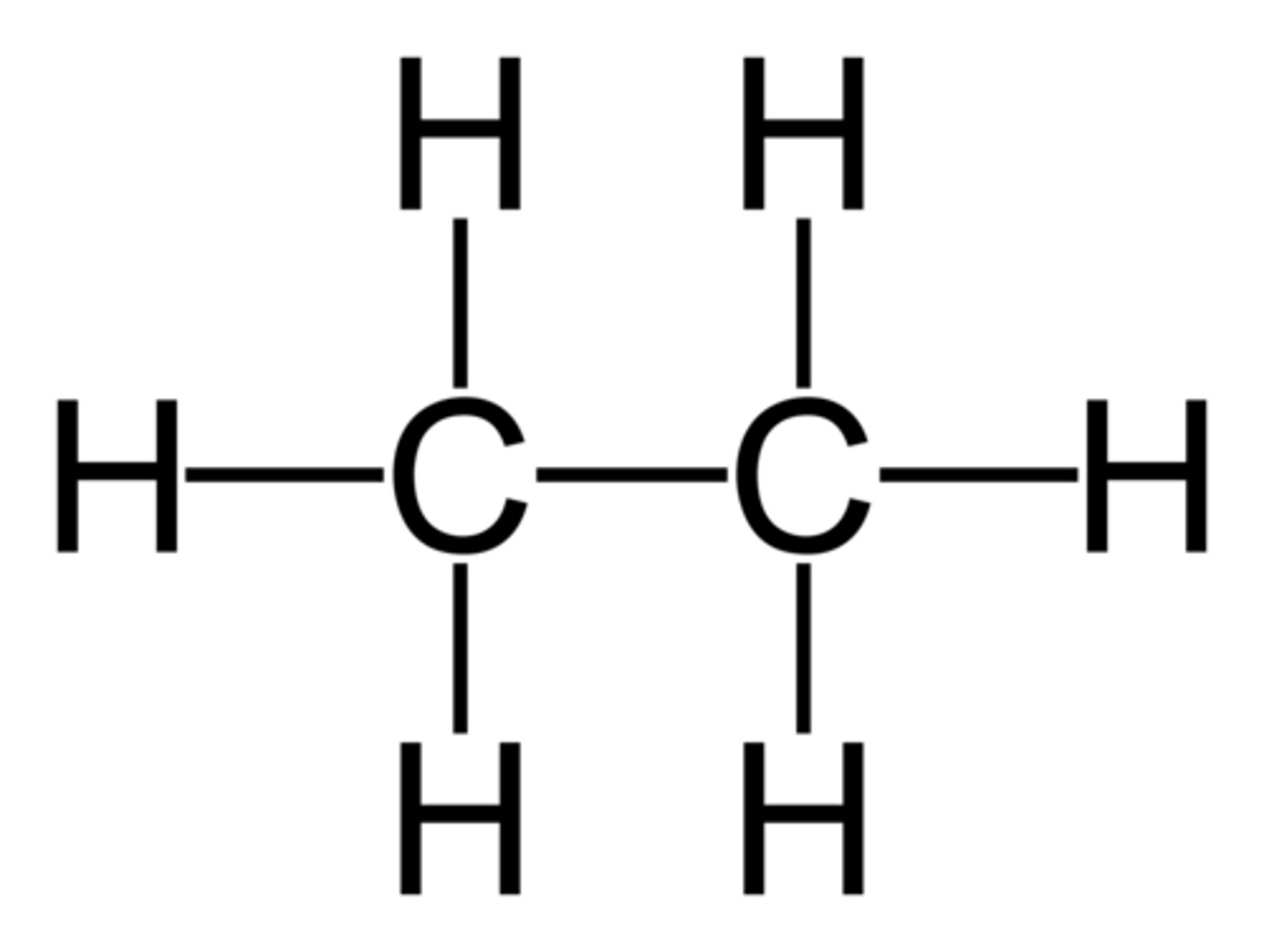
2 things to note when drawing unsaturated HC's
1) dble/triple bonds in a molecule take priority over any branches so MUST be included in the parent chain even if chain would be longer w/o them
^^^as many doubel tripel bonds as possible should be included in parent chain
2) when #ing the parent chain, smallest # MUST go to the double/triple bonds, regardless of any branches
steps to name unsat. HCs when given a diagram
1) identify longest chain that includes dble and triple bonds = parent
2) use the right prefix for the parent based on # of C's and add the suffix -ene for dble bonds and -yne for triple
3) # the parent chain so the doubel/triple bonds have the smallest possibile number
***the FIRST doubel or triple bond in the chain should have the lowest #
3.5) include the location # directly AFTER the parent chain's prefix and directly BEFORE the suffix (ie: hept-2-ene)
****note: the double/triple bond STARTS at the earlier # if its between 2 and 3, its location is written as 2)
4) when multiple of the same bond types, use prefix di, tri, tetra, penta and specify location #
***only example 2 in the picture is correct naming with the location # after the parent's prefix and befor suffix
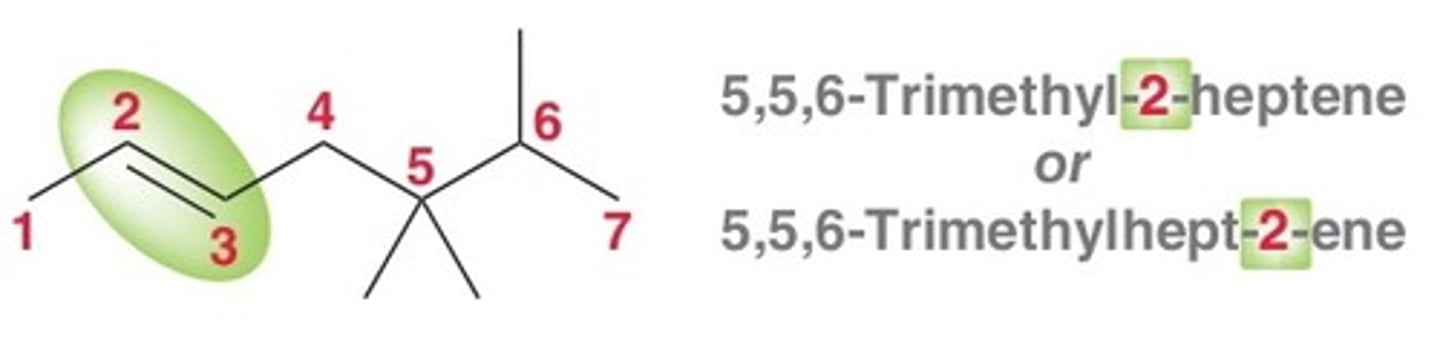
how would the name change if more than 1 double/triple bond is present
you add an "a" to the end of the parent chain's prefix and then add di,tri,tetra,penta after the location number, directly onto the suffix of -ene or -yne
ie) 2,2-tetramethylhexa-2,3-diene
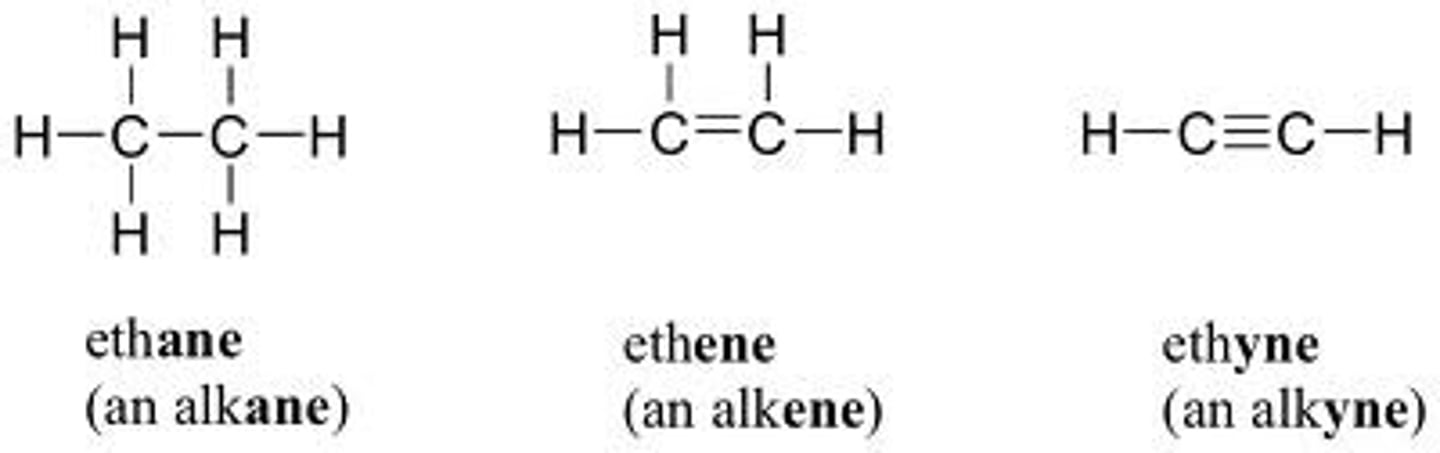
what to note when drawing the line diagram for an alkyne
the shape of a line diagram around a triple bond is always linear NOT zigzag (if triple bond is at 2 to 3, then 1 to 2 and 3 to 4 should be linear lines not zigzag)

how to name if molecule has BOTH double and triple
in alpha order , write the parent prefix then location of doubel bond then suffix (-en) ***not -ene because the "e" ending goes only on the END of a molecules' name
then put dash after en- and do location for triple bond and then - yne
ex: 8,8-diethyldec-3-en-4-yne
what are alkyl halides + how to name + what to note when drawing
molecules with halogen branches (F,Cl,Br, I)
- drop -ide ending of halogen and replace with -o to name branch
**they have no priorty so just treat like othe rbranches and name in alpha order
***when drawing, make sure not to include in parent chain bcs these dont have C's
how to name cycloalkenes or cycloalkynes when 1 dble/triple bond present + how to number
when #ing, you put 1 at the start of the dble or triple bond in the ring and #2 HAS to go on the other end of the double/triple bond
^^ put #1 at whichever end allows #2 to be closer to any branch the ring is connected to
- naming when 1 dble./triple : we dont put location # for the (=) or triple bond bcs always starts at 1, just do cycloprefixene/yne
ie) 3-pentylcyclohexene
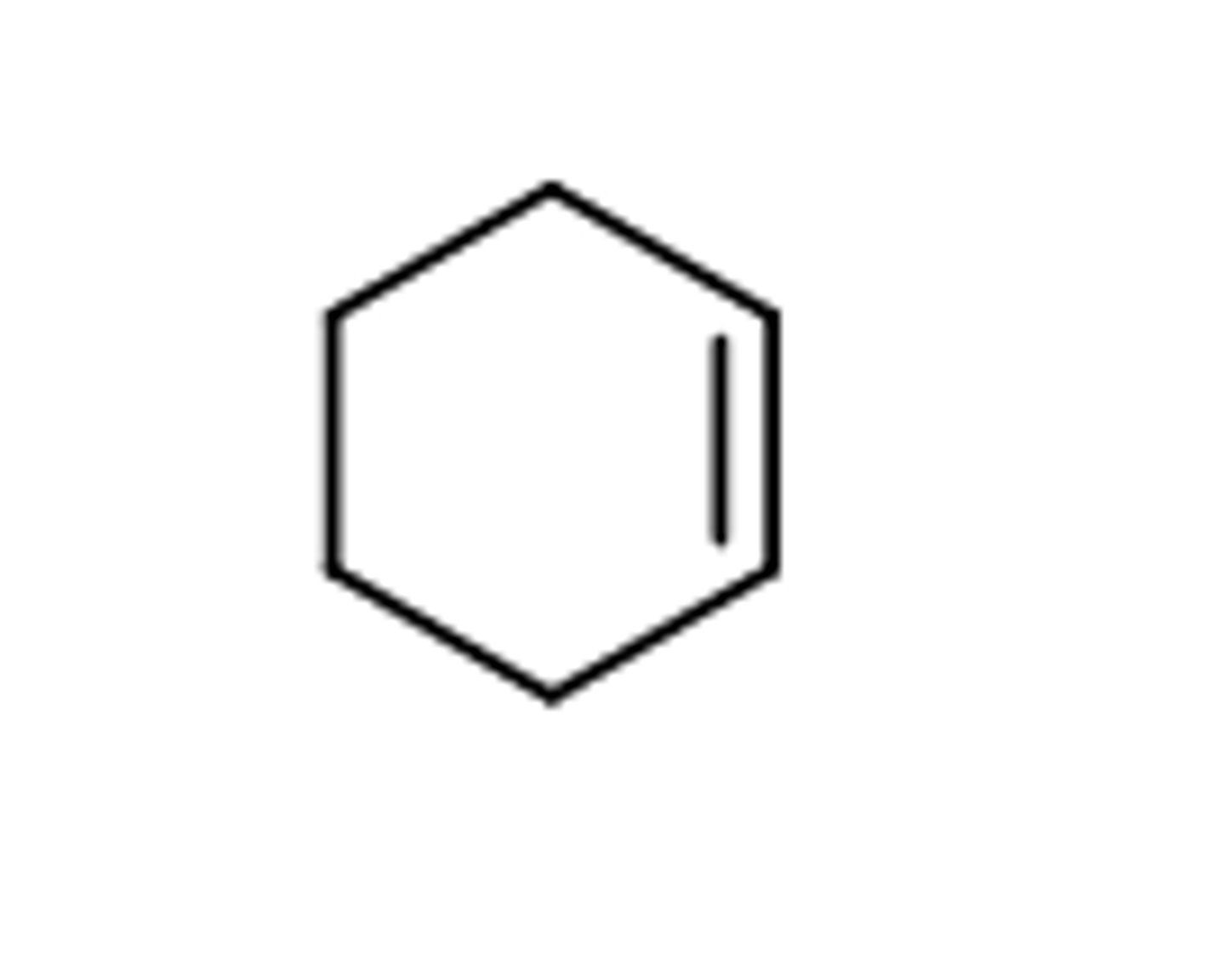
how to name cycloalkenes or cycloalkynes when more than 1 dble/triple bond present + how to best number
naming: cyclo(parentprefix)a-1,3-di,tri,tetraene
ex: 1,2-dimethylcyclohexa-4,5-diyne
to pick which out of the multiple double/triple bond the numbering should start on, pick the bond that will give the lowest # to the FIRST branch coming out of the ring