reaction profiles
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
what must happen for atoms/ particles to react together in a chemical system?
For atoms or particles to react with each other in a chemical system they must first come into contact with each other in a collision
what are the 3 factors for analysing collisions?
Energy
Orientation
Number of collisions per second
what is activation energy?
, the minimum amount of energy required for the collision to be successful, that is for the particles to react together
This minimum amount of energy is called the activation energy and given the symbol Ea
how does activation energy compare in different chemical reactions?
Different reactions have different activation energies, depending on the chemical identities involved
how much energy would a reaction with higher activation require?
Reactions which have higher activation energies require more energy to start than those with lower activation energies
how many reactions have activation energy? why?
All reactions have an activation energy as the chemical bonds in the reactant molecules have to be broken first
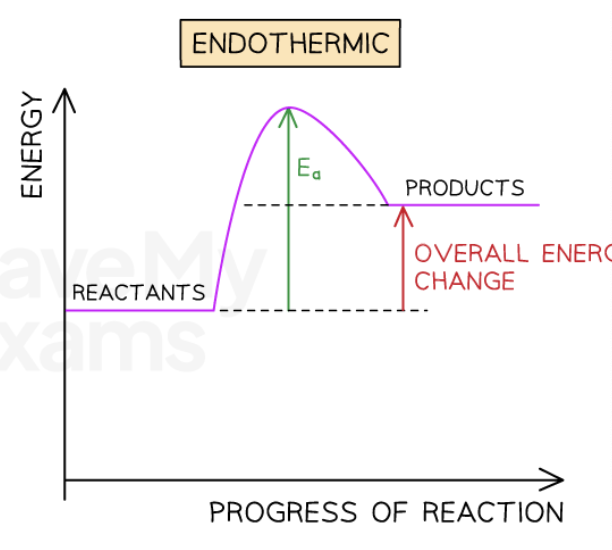
what are energy profiles?
Energy profiles are graphical representations of the relative energies of the reactants and products in chemical reactions
what is displayed on the x and y axis of energy profiles?
The energy of the reactants and products are displayed on the y-axis
the progress of the reaction is shown on the x-axis
what do arrows on energy profiles indicate?
Arrows on the diagrams indicate whether the reaction is exothermic (downwards pointing) or endothermic (upwards pointing)
what represents the overall energy change of a reaction in energy profiles?
The difference in height between the energy of reactants and products represents the overall energy change of a reaction
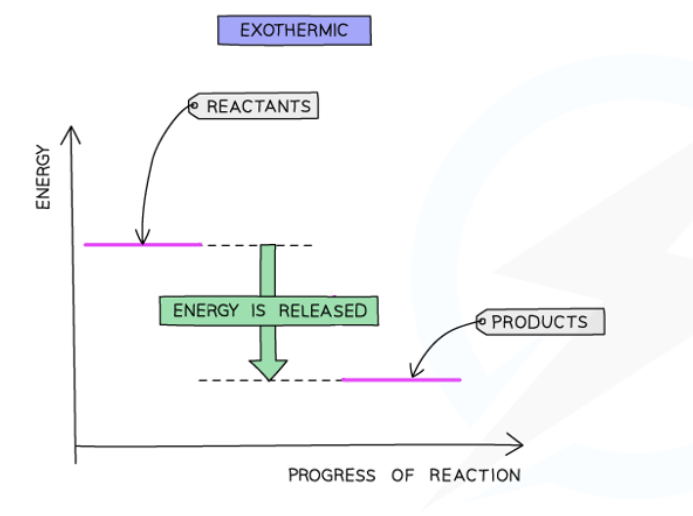
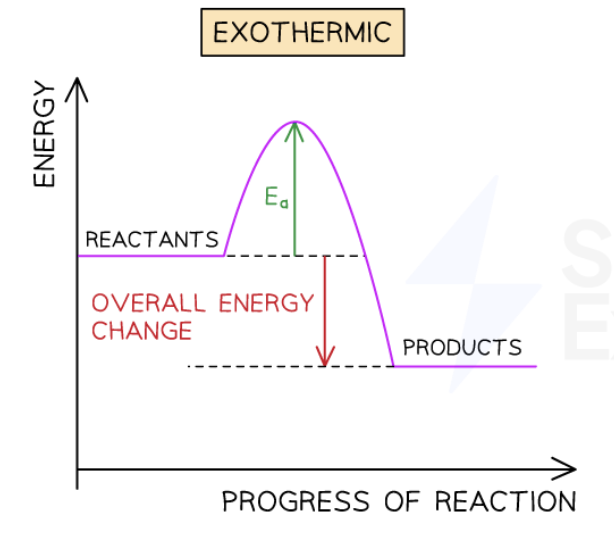
describe this energy profile diagram of this exothermic reaction
Energy is given out in exothermic reactions
The energy of the products will be lower than the energy of the reactants, so the change in energy is negative
This is represented on the energy profile with a downwards arrow as the energy of the products is lower than the reactants
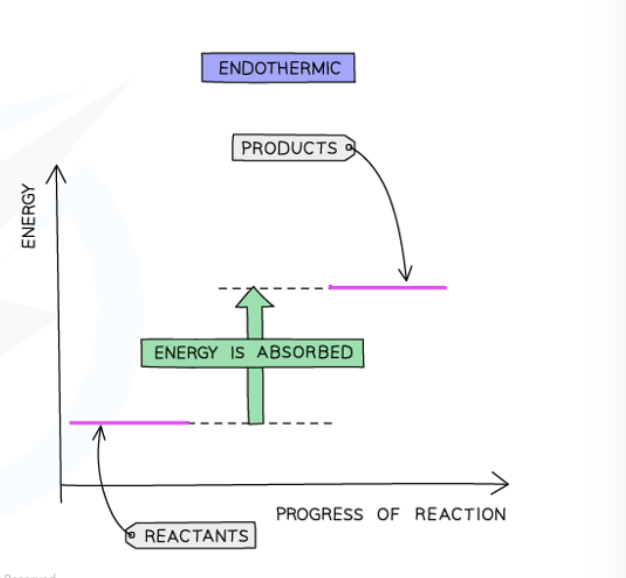
describe this energy profile diagram of this endothermic reaction
Energy is taken in in endothermic reactions
The energy of the products will be higher than the energy of the reactants, so the change in energy is positive
This is represented on the energy profile with an upwards arrow as the energy of the products is higher than the reactants