AP Chem Unit 1
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Law
What happens
Backed by repeated observations
Theory
Why or how something happens
well tested explanation
can change over time
Tera (T)
1012
Giga (G)
109
Mega (M)
106
Kilo (K)
103
deci (d)
10-1
centi (c)
10-2
mili (m)
10-3
micro (μ)
10-6
nano (n)
10-9
Precision
Are results consistent
Accuracy
Is it correct
Random error
unpredictable mistake
Systemic error
consistent mistake
Law of Conservation of Mass
Matter can’t just disappear or reappear
ex. burning wood → turns to ash (mass doesn’t disappear)
Law of Definite Proportions
A compound is always the same recipe
ex. water is always 2 parts hydrogen + 1 part oxygen
Law of Multiple Proportions
Same ingredients, different recipes
ex. C + O = CO or CO2
→ CO = different proportions than CO2 but same elements used
→In CO: 12 g of carbon combines with 16 g of oxygen
→ In CO2: 12 g of carbon combines with 32 g of oxygen
→ The ratio of oxygen masses (16:32) = 1:2, a small whole number ratio
Isotope
Same element, different mass
more neutrons than protons
ex. carbon-12 → 6 protons, 6 neutrons
carbon-14 →6 protons, 8 neutrons
Covalent bonds
when electrons are shared between atoms
Molecule
atoms bonded together
ex. H2O and O2
Compound
molecule but with different elements
ex. NaCl
Ionic bonds
one atom transfers electrons to another atom
atom that loses electron becomes a positive ion (cation)
atom that gains electron becomes a negative ion (anion)
Nonmetal + Nonmetal
Covalent
Metal + Nonmetal
Ionic
Diatomic (covalent)
HONClBrIF
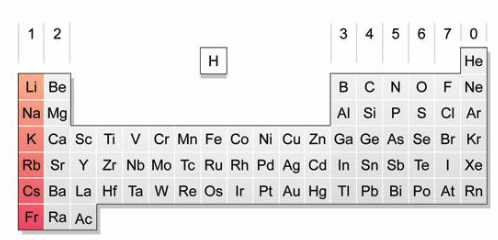
Alkali Metals
+1
Highly reactive
Shiny metals
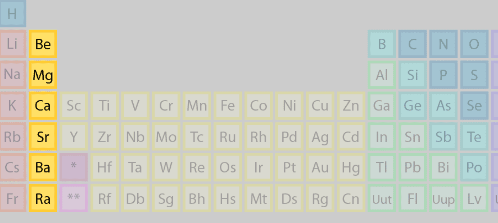
Alkali Earth Metals
+2
Reactive
Transition Metals
Less reactive than groups 1 and 2
Malleable

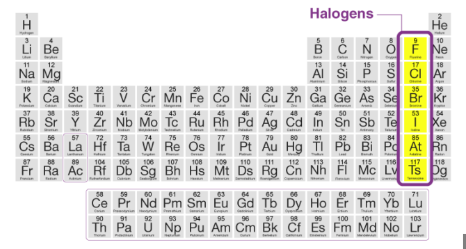
Halogens
-1
Very reactive nonmetals
Noble gases
Nonreactive
Colorless, odorless gases