Second messengers
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
What us cAMP?
First messenger
Discovered in 1951 by Earle Sutherland
What are the main features of cAMP?
Present at low cons in resting calls by adenylate cyclase
Made rapidly when there's agonists
Binds to regulatory subunits PKA
Destroyed by phosphodiesterase - overproduction kills
What happens when protein kinase A binds to cAMP?
Activates catalytic subunits
Phosphorylates targets on serine/threonine residues
Residues = enzymes, receptors, ion channels, TFs
What happens to cAMP in the body?
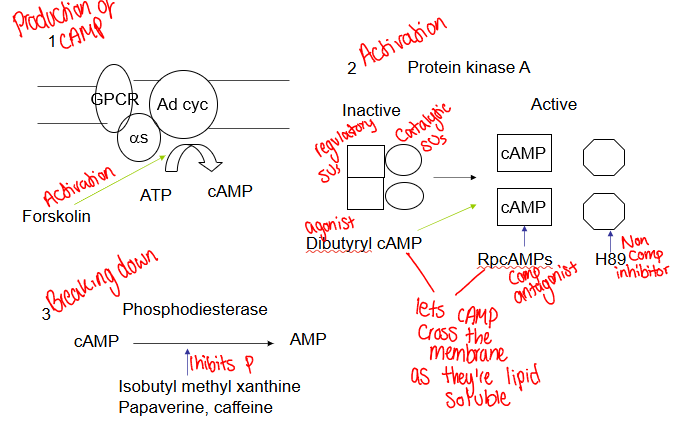
How do you determine whether a drug acts via cAMP?
Agent ↑ cAMP
Mimicked by dibutyryl cAMP and forskolin
Blocked by inhibitors of PKA
Potentate by phosphodiesterase inhibitors (IBMX)
What are the actions of cAMP?
Inhibition of smooth muscle contraction
Stimulation of Ca2+ pump
Activate cardiac Ca2+ channels
Activation of potassium channels
Uncoupling of G-proteins from receptors
Stimulation of protein synthesis
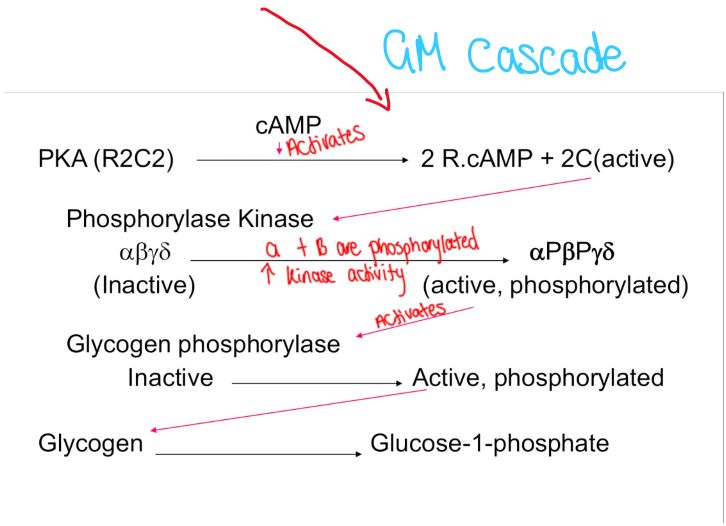
Increased glycogen metabolism
Glycogen metabolism cascade
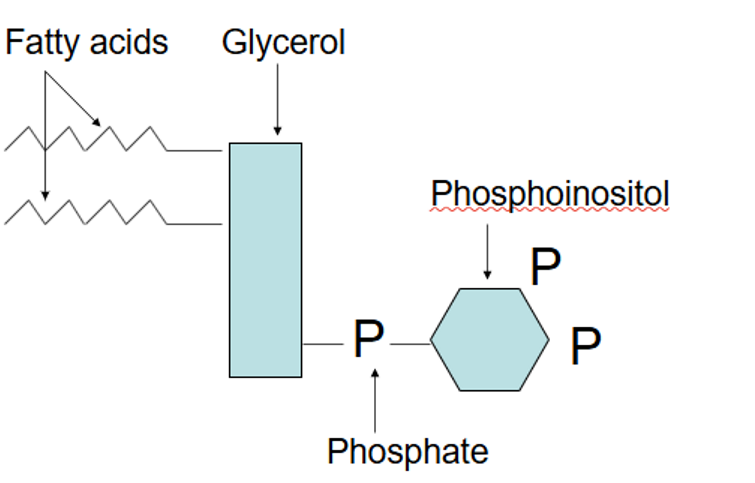
What are phosphoinositides?
Membrane phospholipids based on inositol
Metabolised to yield several 2nd messengers and signalling molecules
Stimulates Ca2+
Increases IP3, protein kinase C (DAG) and tyrosine kinases (PIP3)
Diacylglycerol
Why do hormones need secondary messengers?
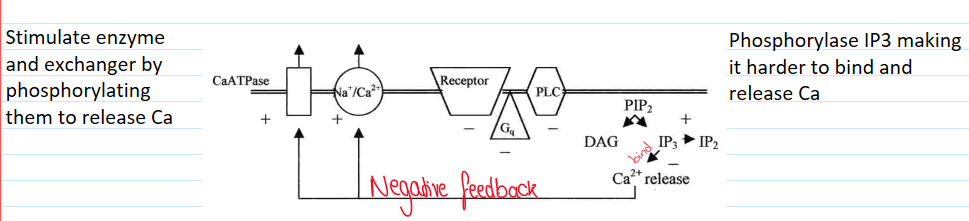
Hormones need a 2nd messenger to release Ca2+ from cytoplasmic stores
Inositol 1,4,5 trisphosphate (IP3) made from phosphatidylinositol 45 bisphosphate hydrolysis (PIP2)
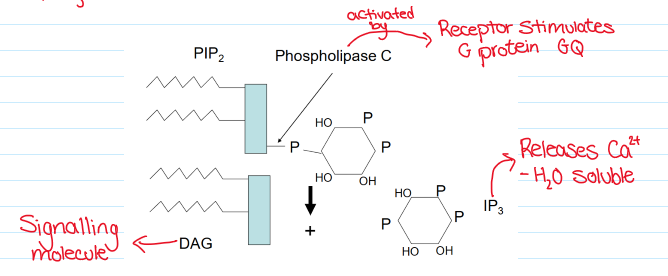
IP3 metabolism
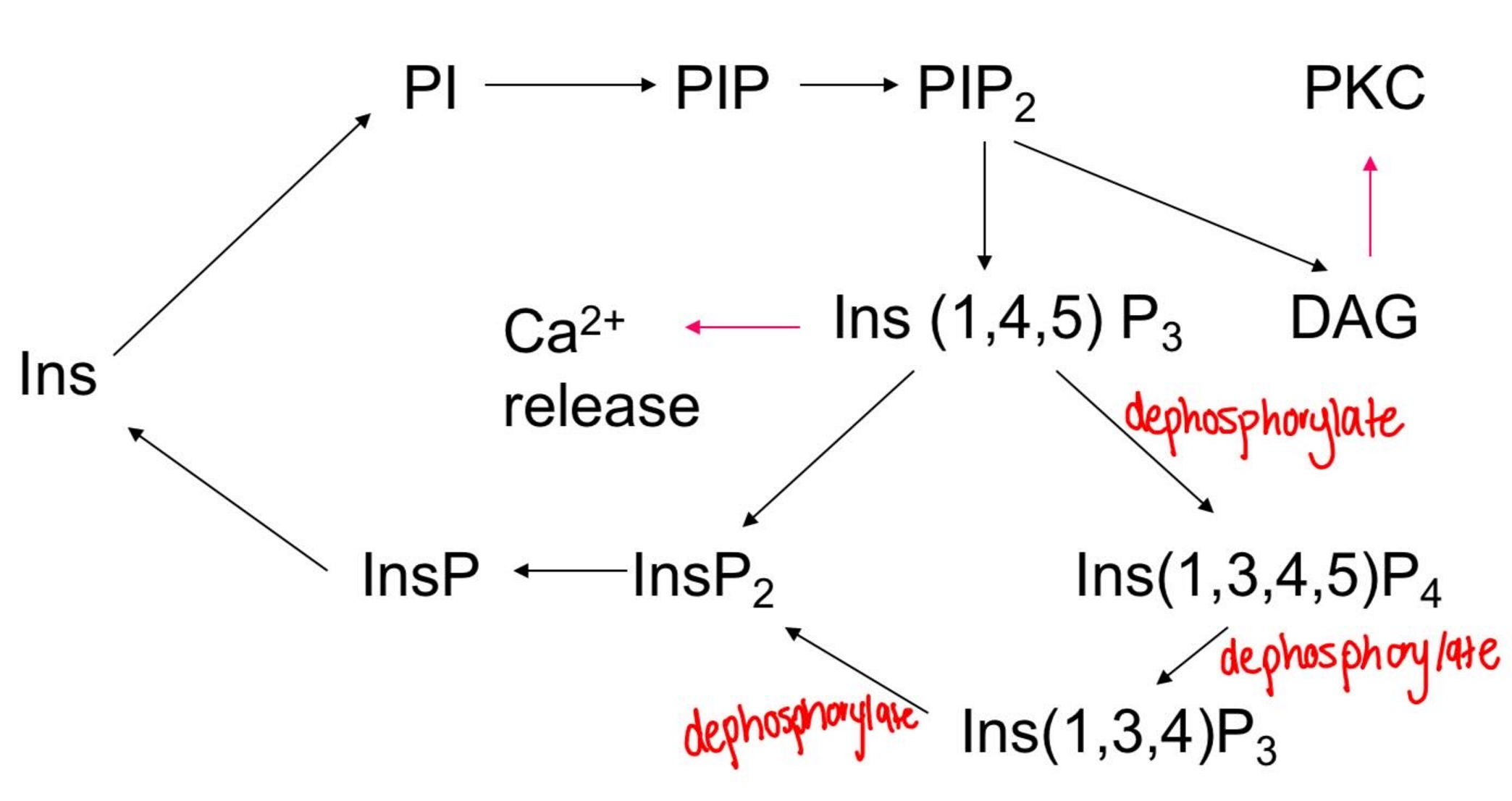
Why is IP4 made?
Stores that can release IP3 have small amounts of Ca2+
External Ca2+ is needed
IP4 binds to a Ca2+ channel to ↑ store
What are docking molecules?
They attract signalling proteins that have the right recognition (pH) domains to the plasma membrane
PIP2 and PIP3
How is PIP3 made?
PtdIns(4,5)P2 can be phosphorylated by phosphatidylinositide-3-kinase (PI3-K) to give PtdIns(3,4,5)P3, or PIP3
Stays membrane associated but can activate some kinases and scaffolding proteins
What are the roles of calcium ions?
Activates ion channels, kinases, metabolic enzymes, structural proteins
Membrane potential changes, secretion, shape changes, cell division, differentiation and intermediary metabolism apoptosis
Where can calcium ions be found?
Excitable cells, via voltage gated channels
From IP3 releasable Ca2+ stores
From other internal stores (eg sarcoplasmic reticulum, cNADPH)
Via store-operated channels (IP4)
Via receptor-gated ion channels (NMDA receptors)
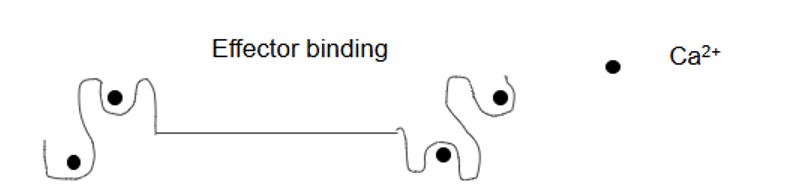
What is calmodulin?
Globular protein, intracellular Ca2+ receptor, 16Kda, binding 4 Ca2+ ions with low µM affinity
How does calmodulin work?
Ca2+ binds
Changes shape
Effector binding site for other proteins
What is calmodulin kinase (CAMK) and specifically CAMKII?
Activates Ca/calmodulin kinase I and II
CAMKII - regulation of receptor density in synapses (memory)
Phosphorylation so it stays active after Ca levels go back to normal
Activation CAMKII
Nerves more closer
Stronger synapse
More communication
What activates protein phosphatase (PP2B) and what happens?
CAM activates it
Dephosphorylation of TF NFAT
- Activates NFAT as there’s no P to stop it from going into nucleus to bind
Cause cytokine production leading to immune response
Why is PP2B inhibition important?
Inhibited by cyclosporin
Stops the body immune system from attacking transplant organs by binding to cyclophilin
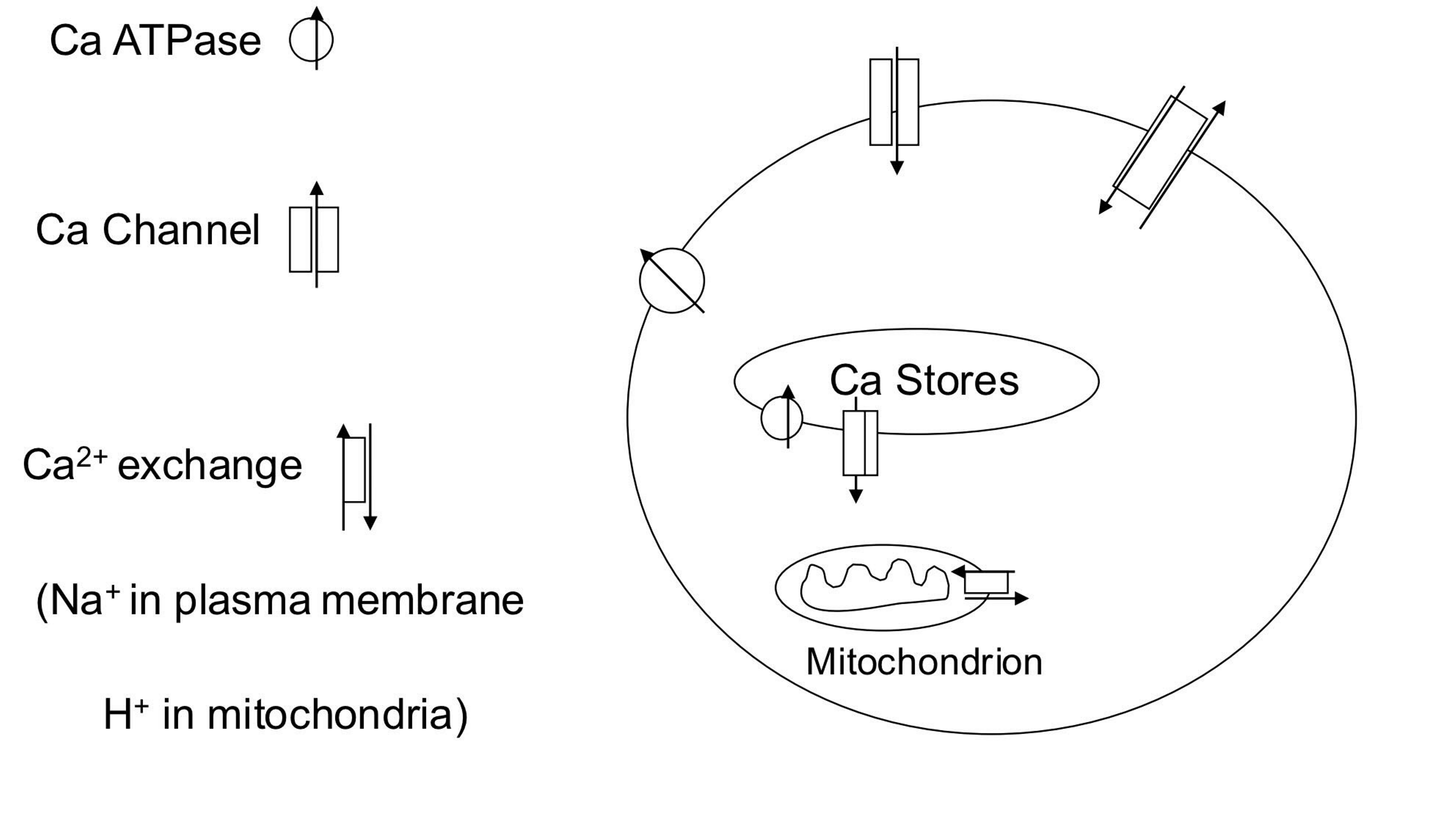
Describe Ca2+ homeostasis
1) Ca2+ enters through voltage and ligand gated ion channels; from intracellular and extracellular stores.
2) It can be removed back into the stores, into mitochondria or out of the cell across the plasma membrane.
3) Removal is via Ca2+ATPase (Ca2+ pump) or Na+/Ca2+ exchange (ie sodium entry down its concentration ground provides the energy for Ca2+ removal against its concentration gradient.
4) Blockers of these processes increase intracellular calcium and can lead to inappropriate cell activation or cell death. Release of Ca2+ from mitochondria is a major means of cell death in apoptosis
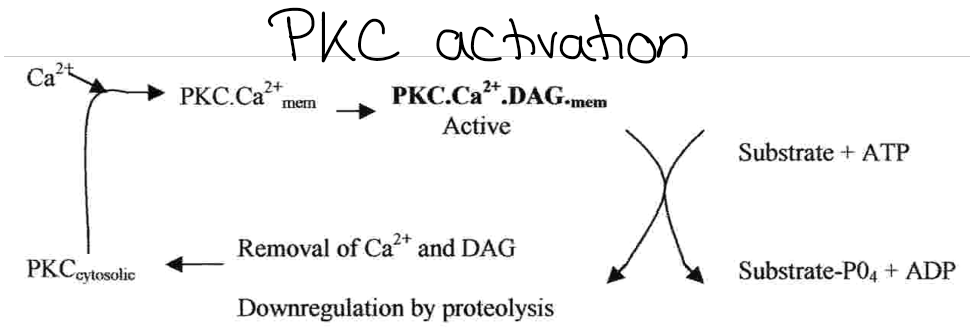
Describe protein kinase C
Multimember family
Serine/threonine kinases usually activated by PtdIns(4,5)P2 breakdown.
All members of the family need phosphatidylserine (PS) and diacylglycerol (DAG) or a free fatty acid (eg arachadonic acid) for activity.
Ca2+ needed by some members
Can phosphorylate GPCRs
Protein kinase C activation
How does protein kinase C affect calcium ion homeostasis?
Reduces receptor activity
Negative feedback loop
What is nitric oxide?
Small, gaseous lipophilic signalling molecule
Endothelial-derived relaxing factor in blood vessels
Functions as a 2nd messenger, NT and autocoid (locally released factor)
Physiological and pathological processes
Biosynthesis of nitric oxide
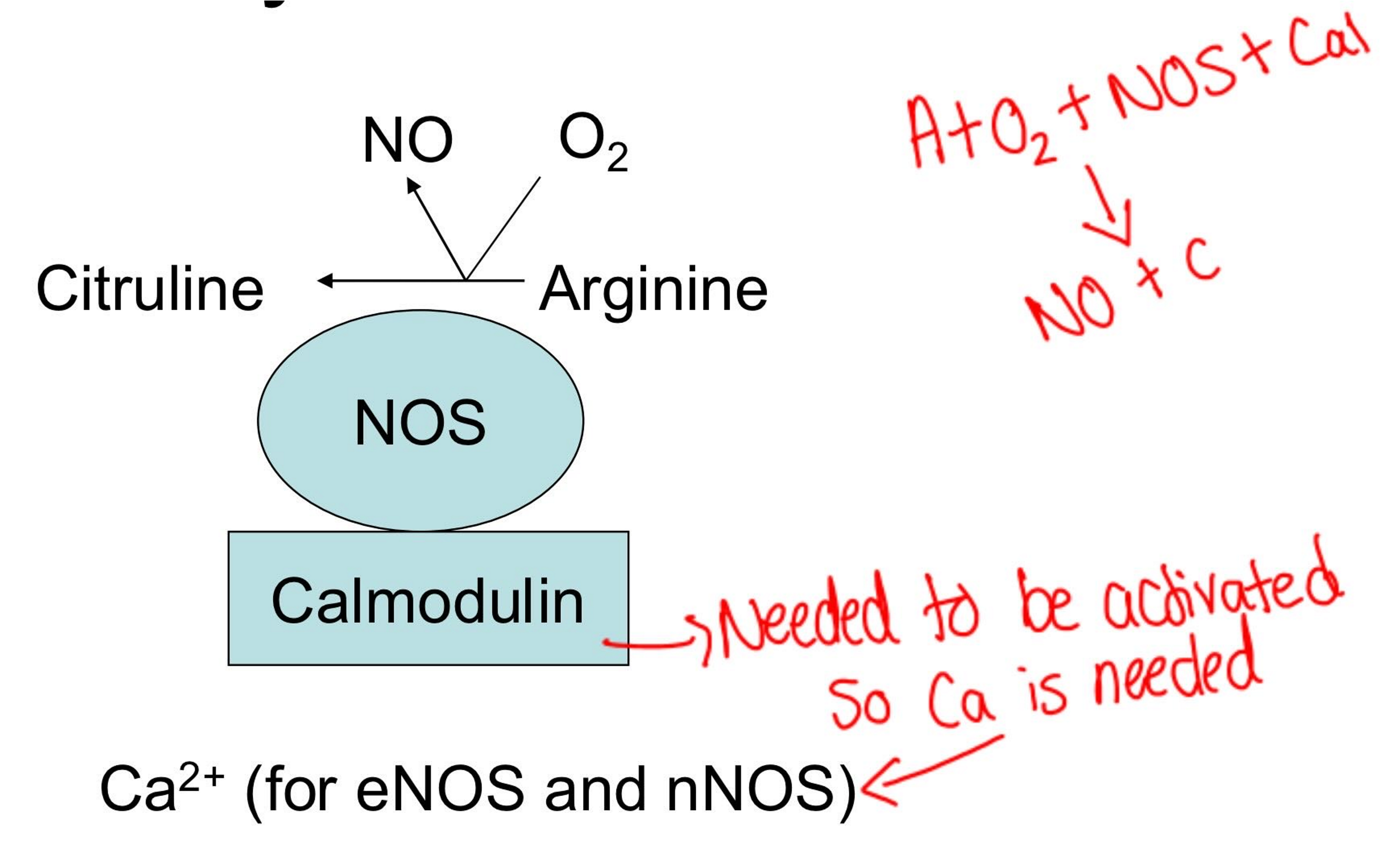
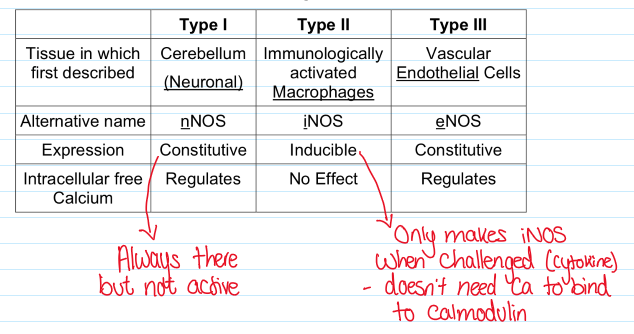
Describe the properties of the 3 types of NOS isozymes
Describe nitric oxide biochemistry
Reactions with iron
Main target is guanylate cyclase (converts GTP to cGMP)
What does cGMP do?
Activates ion channels, protein kinases, inhibits
Stimulates cAMP phosphodiesterase
May produce cADP-ribose (Ca2+ release)
Describe the toxicity of nitric oxide
Free radical
Reacts with superoxide anion to give peroxoynitrite and hydroxyl radicals (reactive and toxic)
Bind to non-haem iron in proteins, leads to destruction and cell death (many are in mitochondria)
What are the roles of nitric oxide and its effect on the body?
Muscle relaxant; cGMP activates protein kinases and ion channels causing decreased contractility and hyperpolarisation.
Non-adrenergic, non-cholinergic (NANC) transmitter. In the CNS involved in long term potentiation and inhibition (LTP, LTD).
From endothelial cells regulates blood pressure and tissue perfusion (cardiac perfusion, erectile tissue). Inhibits platelet aggregation and adhesion.
Cell defence mechanisms. Production stimulated by bacterial endotoxins and cytokines
Describe the pharmacology of nitric oxide
NO donors–Nitro-vasodilators; Sodium nitroprusside
NOS inhibitors– L-NG-nitroarginine
GC inhibitors methylene blue
PDE inhibitors (Type V specific)–IBMX; Dipyridamole; Zaprinast; Sildenafyl (Viagra)