Practice Problems
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Which species below does bleach contain that explains
why it is so effective at disinfecting surfaces?
a) Fluoride
b) Sulfuric acid
c) Chlorine
d) Metal oxides
C
Which of the following statements about sulfuric acid is NOT true?
a) A key intermediate in its formation is elemental sulfur.
b) It is primarily used in the wet method to solubilize
phosphate in rock
c) It is the most manufactured chemical worldwide.
d) The reactions that produce it are primarily reductions.
D
In the reaction below of permanganate anion with
iodide in acidic solution, what has been reduced and
what is the change in oxidation number?
2 MnO4 – + 16 H+ 10 I– → 2 Mn2+ + 8 H2O + 5 I2
a) Manganese, from +7 to +2
b) Iodine, from -10 to -5
c) Oxygen, from -1 to -2
d) Iodine, from -1 to 0
A
Silver is plated on copper by immersing a piece of copper into a solution containing silver (I) ions. In the plating reaction, copper…
a) Is oxidized and is the oxidizing agent
b) Is reduced and is the reducing agent
c) Is oxidized and is the reducing agent
d) Is reduced and is the oxidizing agent
C
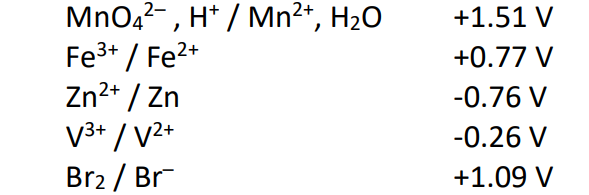
Practice Exam Question
Given the set of standard potentials for the half-cells below,
which is the strongest oxidizing agent?
a) Br2
b) Fe3+
c) MnO42–
d) Zn2+
e) Mn2+
C
Balance this redox reaction in neutral water. What is the coefficient on silver?
Cu(s) + Ag+ (aq) → Cu2+(aq) + Ag(s)
a) 1
b) 2
c) 3
d) 4
B
Use the smallest set of whole number coefficients to balance this reaction in basic solution. What is the sum of the coefficients?
CrO4 2– + NO2– → Cr3+ + NO3–
a) 25
b) 15
c) 10
d) 20
A
For a battery, the cathode is the (positive / negative) terminal and the electrons flow through the external circuit from (anode to cathode / cathode to anode).
a) negative, anode to cathode
b) positive, cathode to anode
c) negative, cathode to anode
d) positive, anode to cathode
D
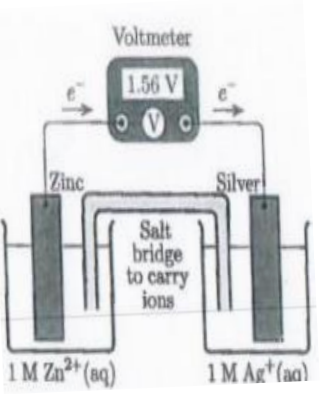
In the electrochemical cell on the
right, what is the cathode?
a) The solid zinc electrode
b) The solid silver electrode
c) The Zn2+ ions
d) The Ag+ ions
B
Sodium is produced by electrolysis of molten
sodium chloride. What are the products of the
anode and cathode, respectively?
a) Cl2 (g) and Na2O (l)
b) Cl2 (g) and Na (l)
c) O2 (g) and Na (l)
d) Na (l) and O2 (g)
e) Cl– (aq) and Na2O (l)
B
As written, which of the following statements about the table of reduction potentials is NOT true?
a) The potentials for ionic species are measured at 1 M.
b) The reactions as presented constitute only half of an
electrochemical cell.
c) The reactions, as written, are all spontaneous.
d) The reactions are all written as reductions.
e) The reference half reaction has a pH of 0.
C
The standard potential of the Cu2+ | Cu electrode is +0.34 V and the standard potential of Pb (s) | Pb2+ is -0.13V
Pb (s) | Pb2+ (aq) || Cu2+ (aq) | Cu (s)
What is the standard potential of the cell, Eo?
a) +0.21 V
b) - 0.47 V
c) + 0.47 V
d) -0.21 V
C
Consider the redox reaction
Cu+2 + 2Ag → Cu + 2Ag+. If all of the reagents have unit activity except for Ag+, which is 10-6 M, which statement below is likely true?
a) This is a new battery
b) E = Eo
c) E = 0
d) Q = K
A
What is the cell potential of the following cell, given the half-cell potentials below?
Pt(s) | Br– (aq, 0.1 M) | Br2 (l) || Au+ (aq, 10-3 M) | Au(s)
Br2 (l) + 2 e– → 2 Br– (aq) E = +1.09 V
Au+ (aq) + e– → Au (s) E = +1.69 V
a) 0.84 V
b) 0.57 V
c) 0.36 V
d) 0.63V
e) 0.60 V
C
A battery formed from the two half-reactions below dies (reaches equilibrium). What is K for the reaction?
Fe2+ → Fe E° = -0.44 V
Cd2+ → Cd E° = -0.40 V
a) 101.33
b) 100.75
c) 102
d) 100.5
A
Taking place in a battery, the reaction below generates a current of 2 A. How much iron is consumed in 5000 seconds?
2 Ag+ (aq) + Fe(s) → Fe2+(aq) + 2 Ag(s)
a) 2.9 g
b) 5.8 g
c) 1.45 g
d) 0.96 g
A
Given galvanic cell below written in standard notation, how many grams of iron are depleted from the anode if the silver cathode increases in mass by 8.0 g?
Fe(s) | Fe2+ (aq) || Ag+ (aq) | Ag (s)
a) 2.1 g
b) 4.2 g
c) 30.9 g
d) 15.5 g
A
Determine the equilibrium constant, K, for the redox reaction below using the table of half reactions. Which of the statements below is true?
2Ag (s) + Fe2+ (aq) ⇌ 2Ag+ (aq) + Fe (s)
i. The sign on the cathode is positive
ii. K = 1.3 x 1042
iii. K = 1.3 x 10-42
iv. Delta G is positive
a) iii only
b) i, ii, iii, and iv
c) i and ii
d) iii and iv
A
An electrochemical cell with an E°cell = +1.2V is a 6-electron process. What is the free energy of this reaction?
a) 720 kJ
b) -72 kJ
c) -720 kJ
d) + 72 kJ
e) -72000 kJ
f) +72000 kJ
C
Which of these statements about metal reactivity is TRUE?
a) Nickel metal is an unreactive metal
b) Zinc metal reacts only in acidic water
c) Mg displaces Cu in a reaction with Cu(NO3)2
d) Sn displaces Mg in a reaction with MgNO3
C
Which of the following is TRUE concerning the engineering of a modern battery?
a) Batteries are made from liquids and gases because it is more affordable.
b) Primary batteries are preferred over secondary batteries in all use cases.
c) It is important to control heat dissipation to improve
efficiency and prevent fires.
d) Efficient batteries use very dense materials to minimize load on transport.
C
Which type of widely used battery is NOT rechargeable?
a) Nickel cadmium (NiCd)
b) Lead-acid
c) Lithium-ion
d) Alkaline
D
The element Mg is used as a _____ for underground pipes by connecting a piece of Mg metal to the pipe with a wire. This method works because Mg is _____?
a) Protective coating, easy to reduce
b) Sacrificial electrode, easy to oxidize
c) Sacrificial electrode, easy to reduce
d) Protective coating, chemically unreactive
e) Protective coating, easy to oxidize
B
What is the atom economy for reaction II with
sodium as the desired product? Which process to
make sodium metal (Na) is greener considering
atom economy?
I) 2NaCl(s) → 2Na(s) + Cl2(g)
II) Na2CO3(s) + 2C(s) → 2 Na(s) + 3CO(g)
a) 20%, I
b) 35%, I
c) 20%, II
d) 35%, II
B