Atoms and elements
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What are the subatomic particles in an atom?
Neutrons protons and electrons
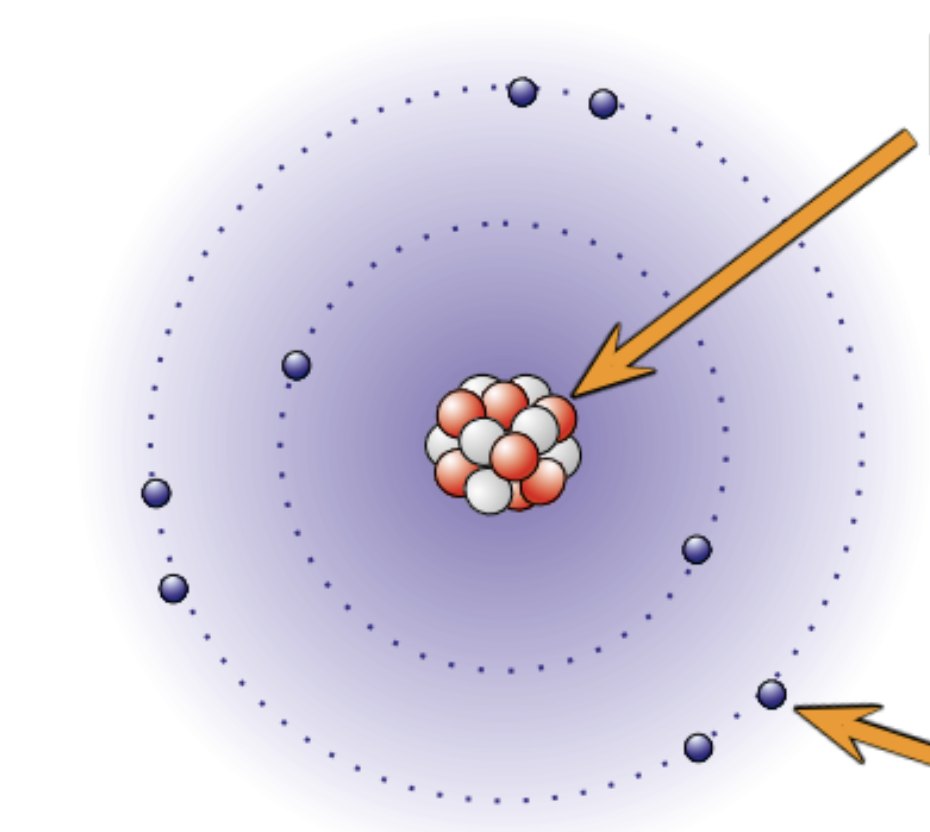
Explain where and what the nucleus is.
In the middle of the atom
Contains neutrons and protons
the radius is about 1 ×10-14
It has a positive charge because of the protons
Almost the whole mass of the atom is concentrated at the nucleus
Explain where and what electrons are?
Move around the nucleus in shells
Negatively charged
The volume of their orbits determine the size of the atom
Electrons has virtually no mass
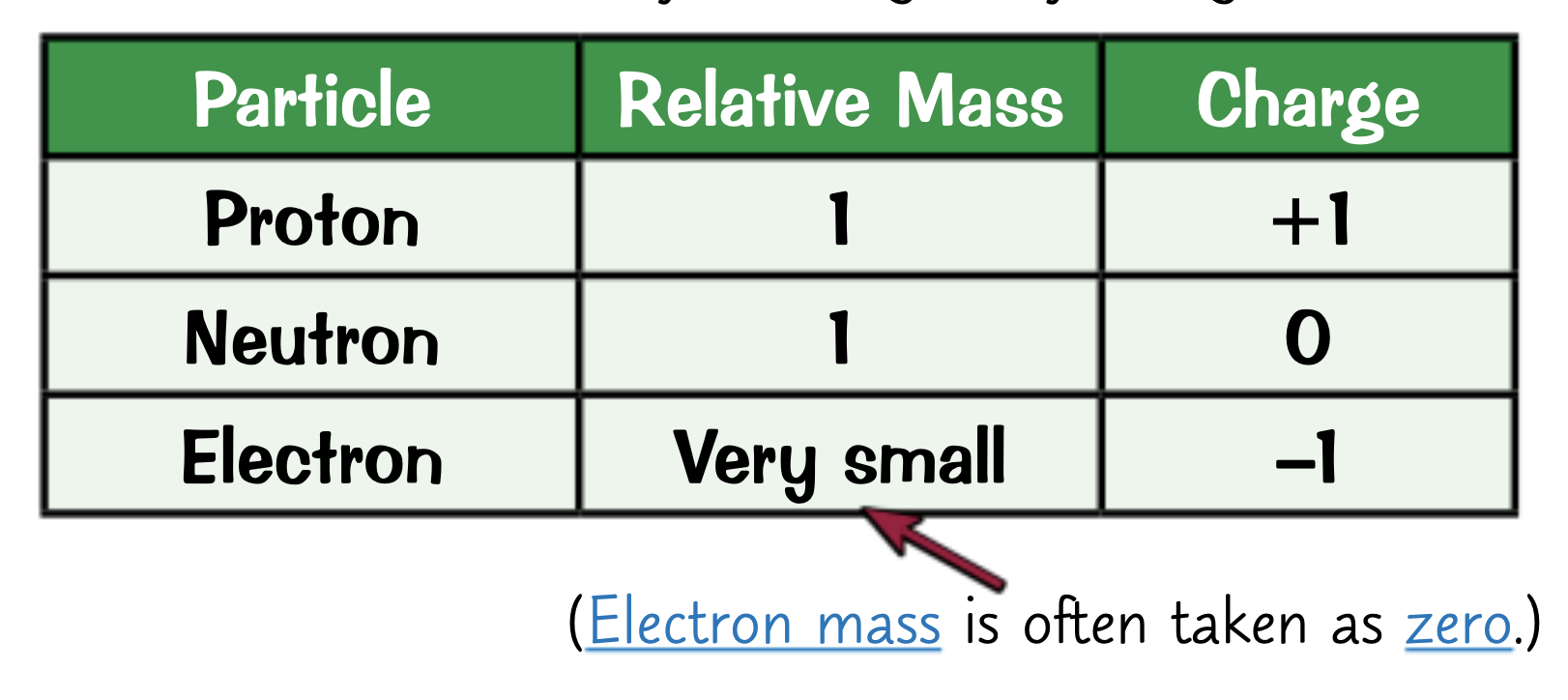
What are the masses and charges of subatomic particles?
Do atoms have a charge?
No they are neutral because they have the same number of protons and electrons
Why do ions have charges?
The proton and electron numbers aren’t equal so the charges do not cancel out
What does the atomic number tell you?
How many protons there are
What does the mass number tell you?
the total number of protons and neutrons in an atom
Where are the atomic number and atomic mass on a nuclear symbol?
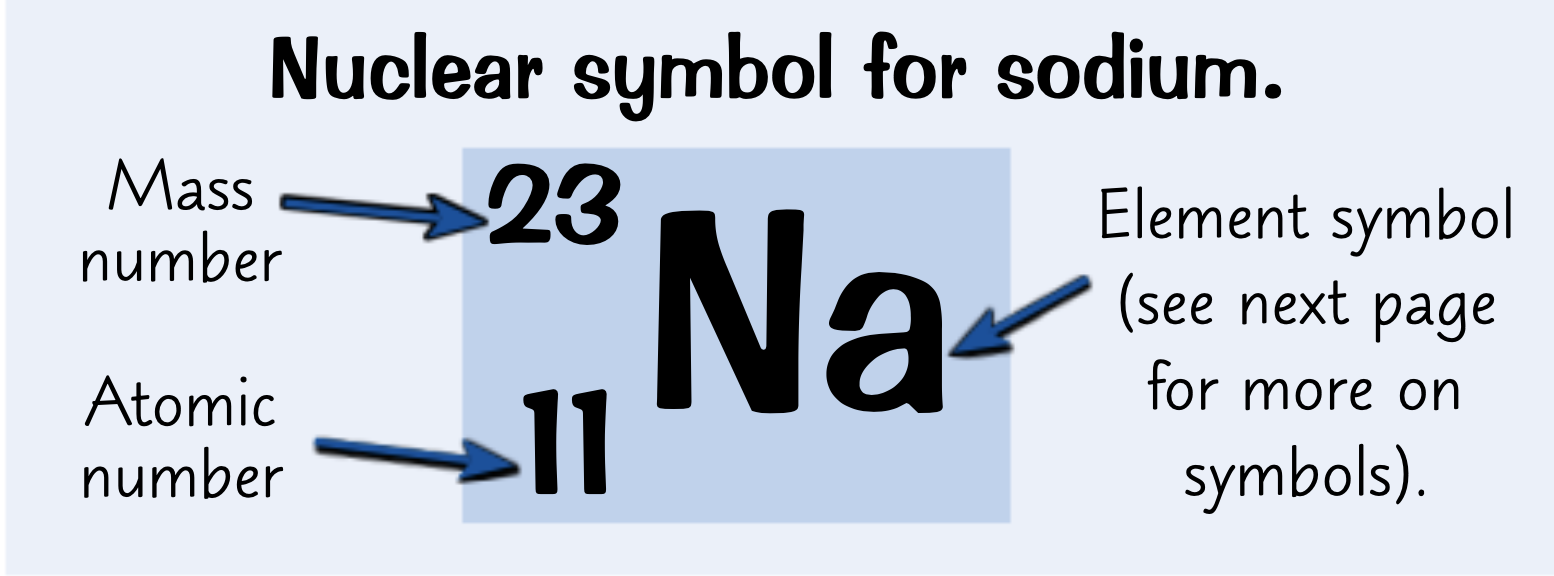
What is the definition of an element?
a substance made up of atoms that all have the same number of protons
What is the role of protons in an element?
Decides what type of atom it is
What is the definition of an isotope?
different forms of the same element, which have the same number of protons but different numbers of neutrons
What is relative atomic mass?
average mass taking into account the different masses and abundances of all the isotopes that make up that element.
What is the formula for relative atomic mass?
Ar = sum of (isotope abundance x isotope mass number) / sum of abundances of all the isotopes
