U9 APES Ocean warming & Acidification
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
Ocean Acidification
The decrease in the pH of the Earth's oceans, caused primarily by the absorption of excess carbon dioxide (CO₂) from the atmosphere.
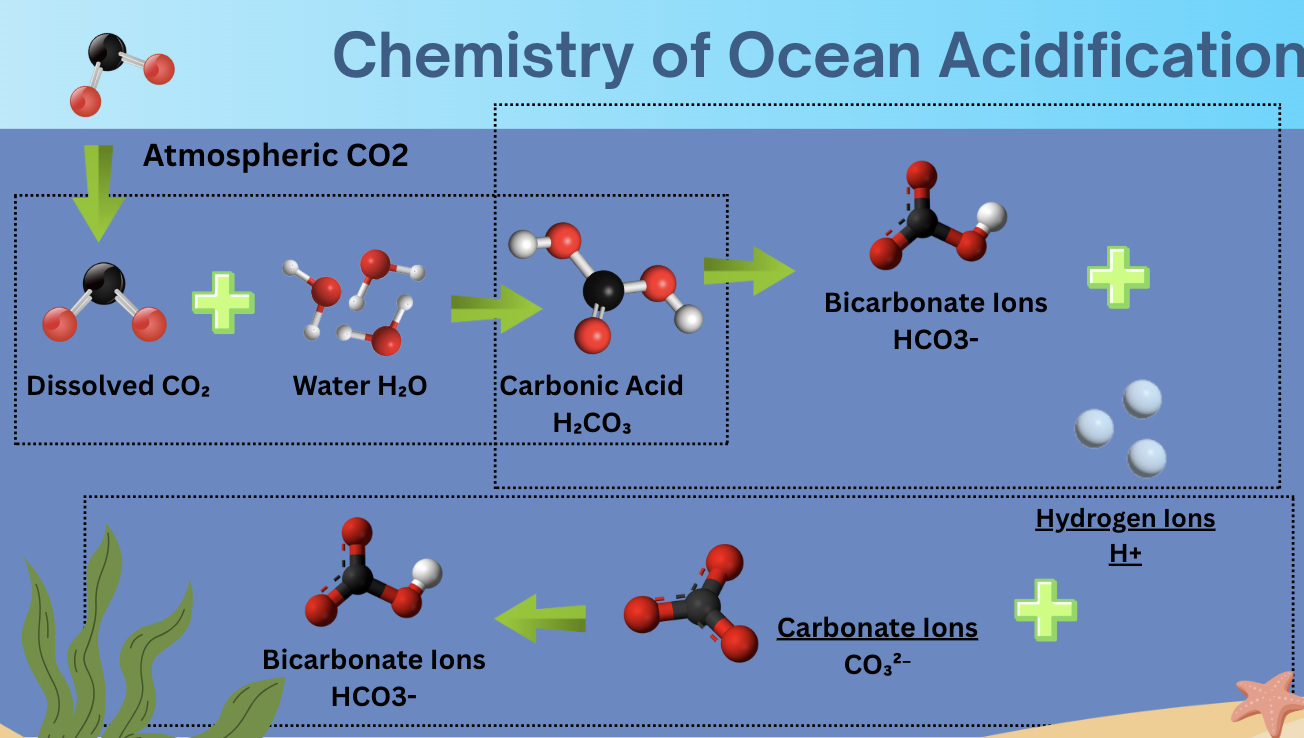
Chemistry of Ocean Acidification
Atmospheric CO2 enters water turning into Dissolved CO2, the Dissolved CO2 combines with the Water (H2O) turning into Carbonic Acid (H2CO3) the Carbonic acid causes the PH in the water to decrease and when is Dissociates it creates the Bicarbonate (HCO3-) and Hydrogen (H+) Ions these ions are able to react with Carbonate Ions (CO3²-) this is a negative change because carbonate ions is needed for the creation of seashells and coral.
Coral Bleaching
A phenomenon in which coral reefs lose their vibrant colors and become white or pale due to stress. The bleaching is a symbiotic process where coral polyps expel the algae (zooxanthellae) that live inside their tissues, which give the coral its color and provide it with nutrients.
Coral Bleaching Cause
Coral bleaching can be caused by rising sea temperatures, Ocean Acidification, Pollution, Solar radiation, disease, Etc.