1.4 - Proteins
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
What is the general structure of an
amino acid?
-COOH carboxyl/ carboxylic acid
group
-R variable side group consists of
carbon chain & may include other
functional groups e.g. benzene
ring or -OH (alcohol)
-NH2 amine/ amino group
Describe how to test for proteins in a
sample.
Biuret test confirms presence of peptide bond
1. Add equal volume of sodium hydroxide to sample at room
temperature.
2. Add drops of dilute copper (II) sulfate solution. Swirl to mix.
(steps 1 & 2 make Biuret reagent)
3. Positive result: colour changes from blue to purple
Negative result: solution remains blue.
How many amino acids are there and
how do they differ from one another?
20
differ only by side ‘R’ group
How do dipeptides and polypeptides
form?
● Condensation reaction
forms peptide bond
(-CONH-) & eliminates
molecule of water
● Dipeptide: 2 amino acids
● Polypeptide: 3 or more
amino acids
How many levels of protein structure are
there?
4
Define ‘primary structure’ of a protein.
● Sequence, number & type of amino
acids in the polypeptide.
● Determined by sequence of codons on
mRNA.
Define ‘secondary structure’ of a protein.
Hydrogen bonds form between O 𝛿-
(slightly negative) attached to ‒C=O & H
𝛿+ (slightly positive) attached to ‒NH.
Describe the 2 types of secondary
protein structure.
α-helix:
● all N-H bonds on same side of protein chain
● spiral shape
● H-bonds parallel to helical axis
β-pleated sheet:
● N-H & C=O groups alternate from one side to the other
Define ‘tertiary structure’ of a protein.
Name the bonds present.
3D structure formed by further folding of
polypeptide
● disulfide bridges
● ionic bonds
● hydrogen bonds
Describe each type of bond in the tertiary
structure of proteins.
Disulfide bridges: strong covalent S-S bonds
between molecules of the amino acid cysteine
● Ionic bonds: relatively strong bonds between charged
R groups (pH changes cause these bonds to break)
● Hydrogen bonds: numerous & easily broken
Define ‘quaternary structure’ of a protein.
● Functional proteins may consist of more than
one polypeptide.
● Precise 3D structure held together by the
same types of bond as tertiary structure.
● May involve addition of prosthetic groups e.g
metal ions or phosphate groups.
Describe the structure and function of
globular proteins.
● Spherical & compact.
● Hydrophilic R groups face outwards & hydrophobic
R groups face inwards = usually water-soluble.
● Involved in metabolic processes e.g. enzymes &
haemoglobin.
Describe the structure and function of
fibrous proteins.
● Can form long chains or fibres
● insoluble in water.
● Useful for structure and support e.g.
collagen in skin.
Outline how chromatography could be
used to identify the amino acids in a
mixture.
1. Use capillary tube to spot mixture onto pencil origin line &
place chromatography paper in solvent.
2. Allow solvent to run until it almost touches other end of
paper. Amino acids move different distances based on
relative attraction to paper & solubility in solvent.
3. Use revealing agent or UV light to see spots.
4. Calculate R f values & match to database
What are enzymes?
● Biological catalysts for intra & extracellular
reactions.
● Specific tertiary structure determines shape of active
site, complementary to a specific substrate.
● Formation of enzyme-substrate (ES) complexes
lowers activation energy of metabolic reactions.
Explain the induced fit model of enzyme
action.
● Shape of active site is not directly complementary
to substrate & is flexible.
● Conformational change enables ES complexes to
form.
● This puts strain on substrate bonds, lowering
activation energy.
How have models of enzyme action
changed?
● Initially lock & key model: rigid shape of
active site complementary to only 1
substrate.
● Currently induced fit model: also explains
why binding at allosteric sites can change
shape of active site.
How could a student identify the
activation energy of a metabolic reaction
from an energy level diagram?
Difference between free
energy of substrate & peak
of curve.
Name 5 factors that affect the rate of
enzyme-controlled reactions.
● enzyme concentration
● substrate concentration
● concentration of inhibitors
● pH
● temperature
How does substrate concentration affect
rate of reaction?
Given that enzyme concentration is
fixed, rate increases proportionally to
substrate concentration.
Rate levels off when maximum number
of ES complexes form at any given
time.
How does enzyme concentration affect
rate of reaction?
Given that substrate is in excess,
rate increases proportionally to
enzyme concentration
Rate levels off when maximum
number of ES complexes form at
any given time.
How does temperature affect rate of
reaction?
Rate increases as kinetic energy
increases & peaks at optimum
temperature.
Above optimum, ionic & H-bonds in 3°
structure break = active site no longer
complementary to substrate
(denaturation).
How does pH affect rate of reaction?
Enzymes have a narrow optimum
pH range.
Outside range, H + / OH - ions
interact with H-bonds & ionic
bonds in 3° structure =
denaturation.
Contrast competitive and
non-competitive inhibitors.
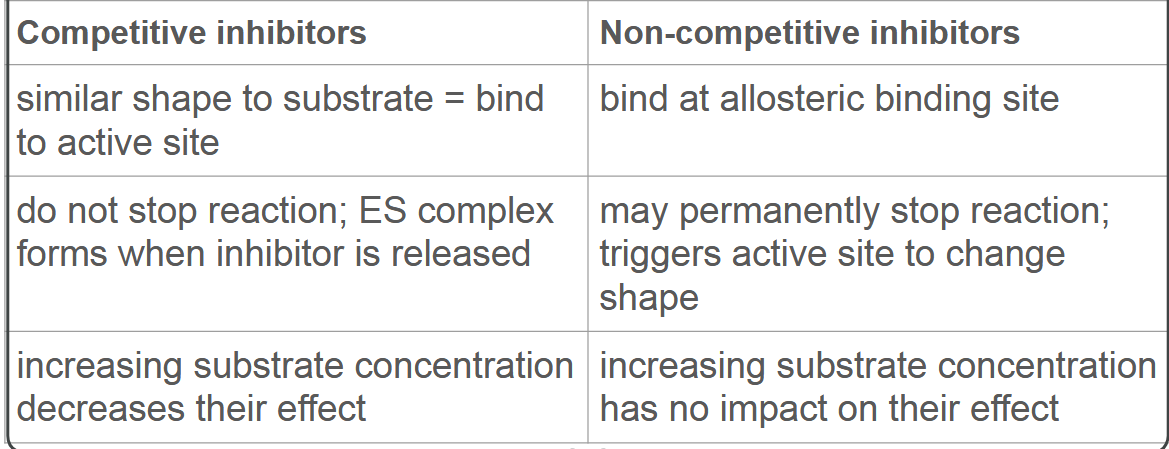
Outline how to calculate rate of reaction
from a graph.
● calculate gradient of line or gradient of
tangent to a point.
● initial rate: draw tangent at t = 0.
Outline how to calculate rate of reaction
from raw data.
Change in concentration of product or
reactant / time.
Why is it advantageous to calculate initial
rate?
Represents maximum rate of reaction
before concentration of reactants
decreases & ‘end product inhibition’