Pathyphysiology of Pain MDR, Pain Management in Horses VCNA, Cornell Pain Video
1/157
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
158 Terms
What is chronic pain classified as?
Pain lasting longer than 3 months from the initial stimulus
Transduction
Process by which a nociceptive stimulus is transferred to the dorsal horn of the spinal cord, where it can undergo modulation, prior to traveling to the thalamus for further processing, and to create the perception of pain
A-delta fibers
Myelinated fibers
Allow rapid impulse conduction
Conduct both nociceptive and non-nociceptive impulses
Distributed at high receptor density in the periphery with a very small receptive field
Activation results in sharp, well-localized pain sensations
Activated by changes int he tissue environment
Have specific receptors that sense mechanical stimulation (“high-threshold” nociceptors), thermal input (heat or cold), and chemical irritation
Map to laminae I and V of the dorsal horn of the spinal cord
A-beta Fibers
Myelinated fibers
Conduct non-nociceptive information under normal circumstances
Important for proprioception
Chemicals that Can Trigger Nociceptive Signals
Increased concentrations of H+ or K+
Histamine
Calcitonin gene-related peptide (CGRP)
PGE2, produced from arachidonic acid by COX
C-Fibers
Unmyelinated so slower transmission rates than A-beta and A-delta fibers
Burning or throbbing pain
Polymodal receptors that respond to changes in temperature (hot or cold), pressure, and pH, and communicate with wide dynamic range neurons
Have a higher activation threshold than A-series fibers
Map to lamina II, also called the substantia gelatinosa (SG)
What mediates visceral pain?
Predominantly C-fiber mediated
Nociception at the Level of the Spinal Cord
Nociception activation triggers the release of neurotransmitters such as glutamate (primarily from A-delta fibers) and substance P (primarily C fibers), and CGRP
Substance P interacts with the neurokinin1 receptor (NK-1)
Glutamate has 3 receptors
Metabotropic glutamate receptor (mGLuR)
a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor
n-methyl-d-aspartate (NMDA)
Calcium channel
Usually inactive due to the presence of a magnesium ion in the channel
With excessive (repetitive or high frequency) stimulation, the Mg is released, and the NMDA channel becomes available to transmit signals from released glutamate
Release of neurotransmitters activates second order neurons, which decussate to the contralateral side of the spinal cord and travel to the brain along spinal tracts
Neospinothalamic Tract
Directs impulses to the thalamus, then to the sensory cortex
Carries impulses primarily from A-delta fibers
Paleospinothalamic Tract
Directs impulses to the peri-aqueductal gray, pons, and medulla, in addition to the thalamus
Carries impulses from primarily C fibers
Spinoreticular Fibers
Carry nociceptive information to the reticular formation, which governs arousal and results in the autonomic and motor responses to pain
Where are signals transmitted to the thalamus projected?
Projected to the somatosensory cortex and limbic system, resulting in the recognition of the presence of pain
Where does modulation of pain occur?
Centrally at the cerebral cortex
Also the level of the spinal cord
Endogenous Modulation of Nociceptive Transmission
Can occur through descending inhibitory tracts from the thalamus and higher areas of the brain or by hormones (e.g. oxytocin)
The descending inhibitory tracts that originate in the periaqueductal gray cause the secretion of serotonin and activation of inhibitory interneurons that modulate the transmission of signals at the level of the SG, through release of the endogenous opioids enkephalin and dynorphin
Agonism of the mu opioid receptor on the afferent neuron prevents neurotransmitter release, while agonism on the secondary neuron results in decreased transmission in the spinal tract
Also a target for exogenously administered opioids
Gate-Control Theory of Pain
Nociceptive input to the dorsal horn decreases activity of inhibitory interneurons, while input from the A-beta fibers (non-painful, proprioceptive sensation) has a stimulatory effect on the inhibitor interneurons (ie. decrease the transmission of nociceptive input through negative interneuron influence)
If the frequency of firing from the A-beta fiber exceeds that of the C-fiber, transmission to the dorsal horn, or to the projection of neurons may be inhibited
Wind-Up
An increased pain sensitivity
Results from prolonged nociceptive stimulation
Wind-Up in the Periphery
A change of the physiologic properties of the nociceptors, such that even non noxious stimuli activate nociceptive input (“peripheral sensitization”)
Wind-Up at the Spinal Cord
Constant release of glutamate results in release of Mg from NMDA receptor ion channels and results in hyperexcitability of dorsal horn neurons (“central sensitization”)
Substance P and calcitonin gene-related peptide (CGRP) release from C-fibers may have an additive effect on NMDA receptor activation
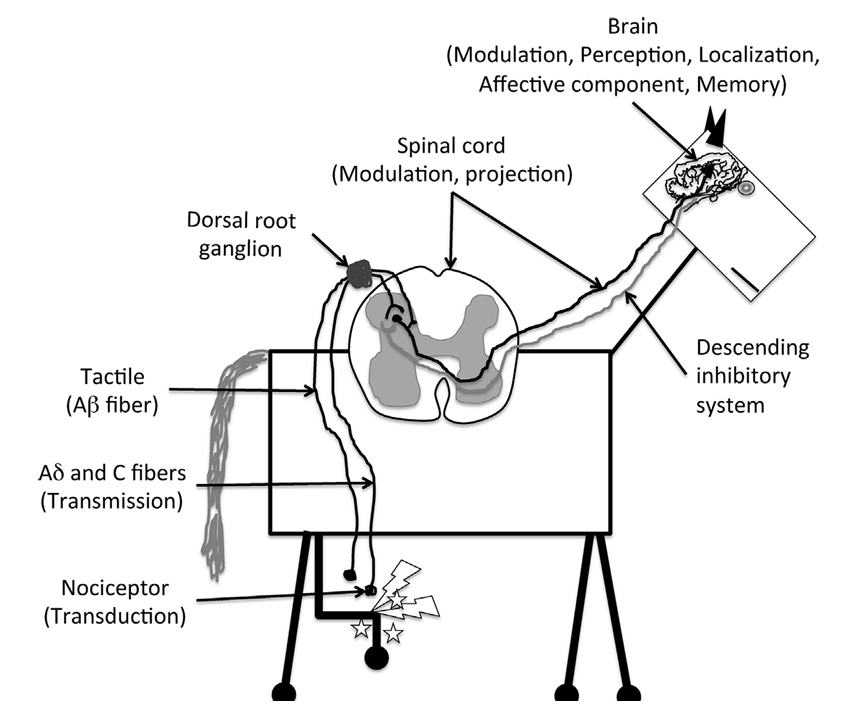
What parts of the pain pathway do nociceptors participate in?
Transduction
What parts of the pain pathway do Ad and C fibers participate in?
Transmission
What parts of the pain pathway does the spinal cord participate in?
Modulation
Projection
What parts of the pain pathway does the brain participate in?
Modulation, perception, localization, affective component, memory
Pain Pathway
Noxious stimuli are recognized by unimodal (recognize a single type of stimulus) or polymodal (recognize multiple types of stimuli) nociceptors located on the terminals of primary afferent neurons (Ad and C) and transduce these stimuli into action potentials
The generated action potentials are transmitted to the dorsal horn of the spinal cord by the primary afferents via the dorsal nerve roots
In the dorsal horn, primary afferents synapse with local excitatory or inhibitory interneurons that modulate the sensory signal and with second-order projection neurons that project the stimulus to the brainstem and thalmus via the spinoreticulothalamic and spinothalamic tracts, respectively
From loci in the brainstem and thalamus, third-order projection neurons relay the signals to the somatosensory cortex, where location and intensity of the stimulus are identified
From the brainstem and amygdala, projection neurons reach the cingulate and insular cortices, which are responsible for the affective/aversive component of the pain experience
Descending pain-moduatory systems exert their modulatory effect predominantly via connections in the dorsal horn through release of neurotransmitters such as serotonin, norepinephrine, and dopamine
The neurotransmitter released and receptor subtype will essentially dictate an antinociceptive or pronociceptive effect
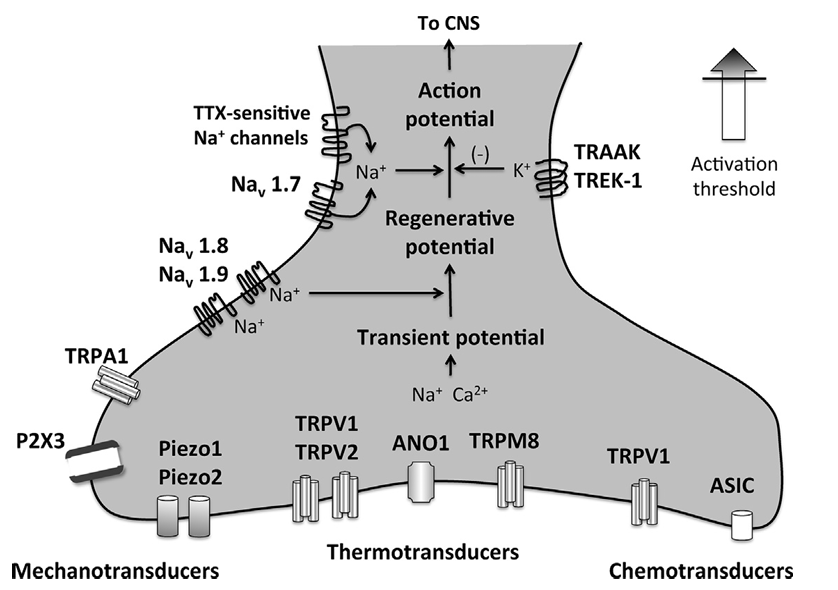
Nociceptive Pain
Produced by activation of high-threshold Ad and C-fiber nociceptors
Peripheral Mechanisms of Nociceptive Pain
Noxious stimuli acts on mechanotransducers, thermotransducers, and chemotransducers
Physicochemical stimuli (pressure, temperature, chemicals) activate ion channels located on sensory nerve endings, creating a transient change in membrane potential that is amplified by sodium channels such as Nav1.8 and Nav 1.9 to form a “regenerative potential”
At this point, endogenous inhibition may occur via activation of potassium channels such as the 2 pore channels TRAAK1 and TREK-1 or further amplified by sodium channels such as Nav1.8 and tetrodotoxin (TTX) - sensitive sodium channels to create an action potential that is propagated toward the CNS where is will be modulated and perceived
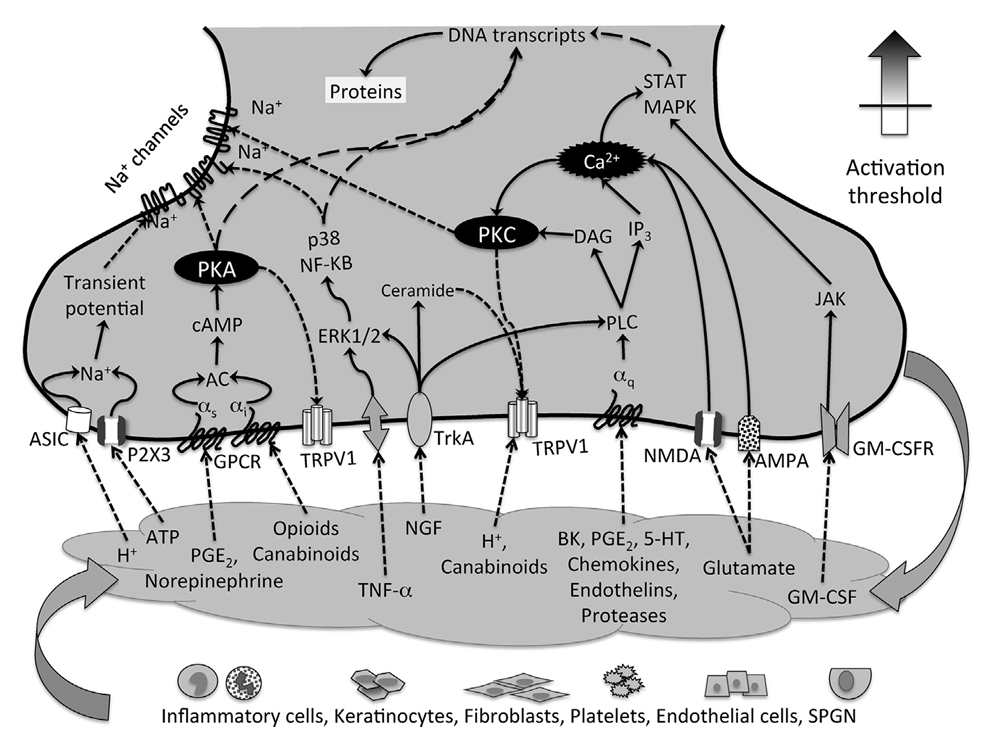
Peripheral Mechanisms of Inflammatory Pain
At the site of tissue injury, inflammatory mediators lead to sensitization of nociceptive (A-d and C-fibers) as well as nonnociceptive (A-beta) sensory afferents
Upon injury, inflammatory and noninflammatory cells release a host of signaling molecules
Mediators such as PGE2, bradykinin, ATP, H+, histamine, serotonin (5-HT), nerve growth factor, TNF-a, IL-6, and others activate ligand-gated ion channels and GPRCs, leading to increases in intracellular ion concentration (Na+, Ca2+), activation of calcium-dependent and cAMP-dependent protein kinases (PKC and PKA), which phosphorlyate membrane ion channels and other GPCRs, lowering their activation threshold
Altered protein trafficking and activation of transcription factors (which translocate to the nucleus located in the cell bodies within the dorsal root ganglion) lead to decreased expression of potassium channels (TRAAK, TREDK-1) and increased expression of sodium channels (Nav 1.8/1.9), contributing to lowering the activation threshold of peripheral nociceptors and increasing afferent excitability
With these changes, nerve endings begin to secrete vasoactive and proinflammatory substances, such as calcitonin-gene-related peptide (CGRP), substance P, and others
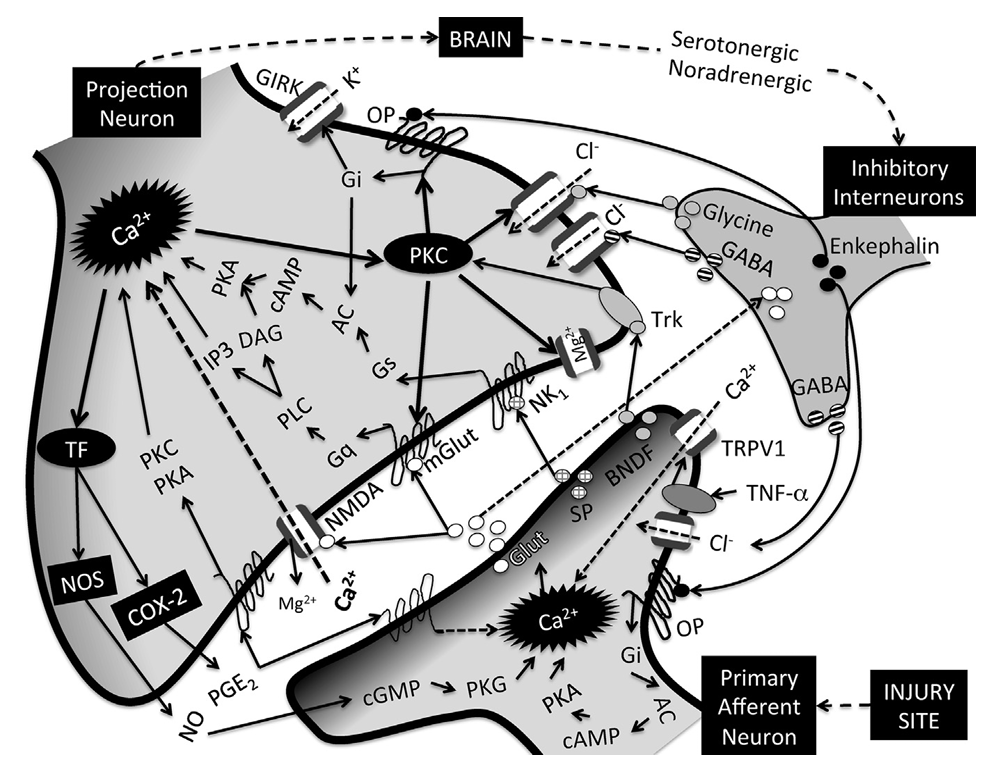
Central Mechanisms of Inflammatory Pain
Action potentials generated at the site of injury and propagated via primary afferents to the spinal cord dorsal horn, lead to activation of voltage-gated calcium channels (VGCC), calcium-dependent pre-synaptic release of neurotransmitters such as glutamate (Glut), substance P (SP), calcitonin gene-related peptide (CGRP), and brain-derived neurotrophic factor (BNDF) and activation of post-synaptic projection neurons as well as inhibitory interneurons
Glutamate is the primary mediator of excitatory neurotransmission, with modulatory influences from SP, CGRP, and BNDF
Inhibitory neurotransmitters include glycine, GABA, and opioid peptides such as enkephalin released by inhibitory interneurons
Decrease pre-synaptic neurotranmitter release and post-synaptic neuronal excitability by activating chloride (Cl-) influx (GABA, glycine), by inhibiting adenylyl cyclase (AC) and thus preventing activation of cAMP-dependent protein kinase (PKA)
Activate G protein-coupled inwardly rectifying potassium (GIRK) channels (enkephalin and other opioids)
Glutamate transporters located on inhibitory interneurons control synaptic concentrations of glutamate
Glutamate released during persistent nociceptive activity activates NMDA (normally blocked by magnesium, Mg2+)and metabotropic glutamate receptors (mGlut), resulting in intracellular calcium responses with subsequent activation of calcium-dependent protein kinase (PKC) as well as activation of transcription factors (TF)
PKC phosphorylates and changes the function properties of ion channels and receptors
Phosphorylation of excitatory receptors (NMDA, mGlut) decreases their activation threshold and increases excitability
Phosphorylation of inhibitory receptors (GABA, glycine, OR) leads to lack of responsiveness to respective ligands and decreased inhibition
Activation of transcription factors induce the synthesis of proteins such as nitric oxide synthase (NOS) and COX-2, with production of NO and PGE2
NO and PGE2 act presynaptically to potentiate neurotransmitter release
PGE2 also acts post-synaptically at different prostanoid receptors to activate PKA and PKC-dependent signaling mechanisms which will contribute to excitability of projection neurons
Excessive uptake of glutamate leads to death of inhibitory interneurons, further contributing to disinhibition
Microglial release of several inflammatory mediators, including TNF-a, lead to transcription changes in pre- and post-synaptic neurons that contribute to increased excitability and long-term potentiation of pain circuits
Unidimensional Pain Scoring Systems
Relatively simple, focusing primarily on pain intesnity without much regard to sensory and emotional qualities of pain
Multidimensional Pain Scoring Systems
More complex, examining the intensity as well as the sensory and affective (emotional) qualities of pain, to provide a more comprehensive assessment of aptient’s pain
Preemptive Pain Scoring (PPS) Pain Scale
Relates the expected level of pain with the invasiveness of the procedure
A degree of pain (none, mild, moderate, severe) is assigned based on this criterion
Simpile and useful for planning perioperative analgesic strategies
Does not take into account individual variation
Not useful in assessing response to therapy
Reliability of Preemptive Pain Scoring (PPS) in Horses
Not tested for reliability but useful for planning perioperative analgesic protocol
Simple Descriptive Scales (SDS) Pain Scale
The most basic pain scale
Usually includes 4 or 5 descriptors from which observers chose level (no pain, mild pain, moderate pain, severe pain, very severe pain)
Simple to use but extremely subjective and discontinuous
Heavily evaluator-dependent
Does not detect small changes in pain behavior
Reliability of Simple Descriptive Scales (SDS) in Horses
Not tested for reliability
Numerical Rating Scales (NRS) Pain Scale
These can include simple descriptive scales with numbers assigned to each pain level for ease of tabulation and analyses (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain, 4 = very severe pain) or it may constitute numbers from 0 (no pain) to 10 (worse pain possible) placed at equal distances along a horizontal line
This implies equal difference or weighting between numbers, which is often not the case, and they are discontinuous scales
Reliability of Numerical Rating Scales (NRS) in Horses
Not tested for reliability
Visual Analog Scale (VAS) Pain Scale
Created in an attempt to improve on discontinuous scales
This scale has been widely used in veterinary medicine
Consists of a continuous line (usually 100 mm) anchored at either end with a description of the limits of the scale (no pain at one end and severe pain at the other end)
The observer places a mark on this line corresponding to the perceived degree of pain in the horse under observation
THe pain score is the distance (in mm) from zero to the mark
Visual Analog Scale (VAS) Reliability in Horses
Tested for reliability in laminitis, colic, and synovitis
In general, it has fair (synovitis) to good (colic) to very good (laminitis) interobserver reliability
Dynamic and Interactive Visual Analog Scale (DIVAS) Pain Scale
An extension of the VAS
Horses are first observed for normal/abnormal behaviors (ie, expression, demeanor, posture, stance and mobility) from a distance undisturbed
They are then approached, handled, and encouraged to walk by offering food/treats or using a lead rope
The painful site and surrounding area are then palpated, and a final overall assessment of pain is made, usually using a 100-mm line as in the VAS
Dynamic and Interactive Visual Analog Scale (DIVAS) Reliability in Horses
Not tested for reliability but presumably at least as reliable as VAS
Used to assess laminitis pain
Composite Pain Scales (CPS)
Further development of the simple descriptive and numerical rating systems
Certain behavior categories are chosen (ie, pawing, kicking at abdomen, appetite, appearance, sweating, posture, and assigned a value according to descriptors of intensity/frequency
Composite Pain Scales (CPS) Reliability in Horses
Many uses
Has been reliably used for assessing abdominal and musculoskeletal pain
Horse Grimace Scale/Pain Face
Focuses on changes in facial expression described as low or asymmetrical ears, angle appearance of the eyes, withdrawn or tense stare, mediolaterally dilated nostrils, and tension of lips, chin, and certain facial muscles
Horse Grimace Scale/Pain Face Reliability in Horses
Used to assess postcastration pain, in 2 experimental models of acute pain (mechanical and chemical), and in horses with acute laminitis
Appears to be effective and reliable for assessing acute pain, but not yet formally validated
Therapies that Target Nociceptors (Transduction)
Local anesthetics
Morphine
Ketamine
Cryotherapy
COX inhibitors
sEH inhibitors
Surgical technique
Acupuncture
Therapies that Target Ad and C Fibers (Transmission)
Local anesthetics
Alchohol
Buprenorphine
Ketamine
Xylazine
Therapies that Target the Spinal Cord (Modulation, Projection)
Opioids
Alpha-2 agonists
Ketamine
COX inhibitors
sEH inhibitors
Tramadol
Benzodiazepines
Gabapentin
Acupuncture
Therapies that Target the Brain (Modulation, Perception, Localization, Affective Component, Memory)
General anesthetics
Opioids
Alpha-2 Agonists
Treatment Options for Nociceptive Pain
Local or general anesthetics
Opioids
Alpha-2 agonists
Acupuncture
Treatment Options for Inflammatory Pain
COX inhibitors
Soluble epoxide hydrolase inhibitors
Local anesthetics
Acupuncture
Opioids
Fish oil
Cryotherapy
Tiludronate
Polyphenols (ie reservatrol)
Weight management
Treatment Options for Neuropathic Pain
Tramadol
Ketamine
Soluble epoxide hydrolase inhibitors
Gabapentin
Local anesthetics
Fish oil
Cyproheptadine
Tiludronte
Polyphenols (ie, resveratrol)
Acupuncture
Cytotoxicity of Local Anesthetics from Least to Most
Ropivacaine, mepivacaine, lidocaine, bupivacaine
Definition of Pain
An unpleasant sensory and emotional experience associated with actual or potential tissue damage
Acute Pain
Occurs immediately after a stimulus is received
Severity can vary
Responds well to treatment
Subsides once stimulus is removed
Chronic Pain
Persists past initial stimulus (3-6 months)
Severity can vary
May or may not respond well to treatment; may require a “multi-modal” approach
Can result in allodynia, hyperalgesia, and opioid tolerance
Nociception
Stimulation of sensory nerve cells called nociceptors, which produce a signal that travels along a chain of nerve fibers via the spinal cord to the brain
First Neuron in the Nociceptive Pathway
Primary afferent neuron
Transduction of noxious stimuli and conduction of signals from the peripheral tissues to neurons in the dorsal horn of the spinal cord
Second Neuron in the Nociceptive Pathway
The projection neuron
Receives input from the primary afferent neurons and projects to neurons in the medulla, pons, midbrain, thalamus, and hypothalamus
Third Neuron in the Nociceptive Pathway
Supraspinal neurons
Integrate signals from the spinal neurons and project to the subcortical and cortical areas where pain is finally perceived
Where are visceral nociceptors located?
Organs and linings of body cavities
Characteristics of Pain from Visceral Nociceptors
Poorly localized, diffuse, deep, cramping or splitting
Sources of Acute Pain from Visceral Nociceptors
Chest tubes, abdominal drains, bladder, and intestinal distension
Sources of Chronic Pain from Visceral Nociceptors
Pancreatitis, liver metastases, colitis
Where are somatic nociceptors located?
Cutaneous: skin and subq tissues
Deep somatic: blood vessels, muscle, connective tissue
Characteristics of Pain from Somatic Nociceptors
Well-localized, constant, itchy
Sources of Acute Pain from Somatic Nociceptors
Incisions, insertion site of tubes and drains, wound complications, orthopedic procedures, skeletal muscle spasms
Sources of Chronic Pain from Somatic Nociceptors
Bony metastases, arthritis, low-back pain
Where are non-nociceptor or neuropathic receptors located?
Nerve fibers, spinal cord, CNS
Characteristics of Pain from Non-Nociceptor of Neuropathic Receptors
Generalized along distribution of damaged nervous structures
Sources of Acute Pain from Non-Nociceptor or Neuropathic Receptors
Poorly localized, shooting, burning, fiery, shock-like, sharp, painful numbness
Sources of Chronic Pain from Non-Nociceptor or Neuropathic Receptors
Nervous tissue injury due to diabetes, chemotherapy, neuropathies, post-therapeutic neuralgia, trauma, surgery
What are the 4 phases of nociceptive pain?
Transduction
Transmission
Perception
Modulation
Transduction
Substances are released by damaged tissues and lead to the generation of an action potential
Chemical stimuli
Thermal stimuli
Mechanical stimuli
What is the main difference between cytokines and chemokines?
Cytokines - protein that are important for cell signaling, released from the cell
Chemokines - type of cytokine, just there to induce chemotaxis, will attract other types of inflammatory cells
Transmission
Action potential continues from the site of damage to the spinal cord, then ascends up the spinal cord to the higher centers in the brain
What are the peripheral afferent nociceptors?
First order neurons
A-B fibers
A-d fibers
C fibers
What is the ascending pathway for pain and temperature stimuli?
Spinothalamic tract
What is the ascending pathway for touch and proprioception stimuli?
Dorsal column system
What activates A-B nociceptors?
Light touch and/or moving stimuli
Mostly sensory
A-B Nociceptor Conduction Speed
Fast - myelinated
Where are A-B fibers located?
Primarily in skin, normally don’t produce pain
What activates A-d nociceptors?
Mechanical and thermal stimuli
A-d Nociceptor Conduction
Fast - myelinated
What type of pain do A-d fibers produce?
Short-lasting, pricking-type pain
What activates C fibers?
Mechanical, chemical, and thermal stimuli
C Fibers Conduction
Slow - unmyelinated
What type of pain do C fibers produce?
Dull, poorly localized, burning type pain
What are the 2 classes of C nociceptors?
Petidergeric
Nonpetidergic
Peptidergic C Nociceptors
Expresses neuropeptides
Substance P
Calcitonin gene related peptide (CGRP)
Neurokinin A
Expresses receptors
Nonpeptidergic C Nociceptors
Expresses several receptors for neurotrophic factors and ion channels
Ascending Pathways of the Pain Pathway
Nociceptive fibers synapse with second order neuron at the dorsal root ganglion in the dorsal horn of the spinal cord
First order neuron from the nociceptors goes to the dorsal horn and the rexed laminae
Information travels to the contralateral ventral horn (decussation) and then up the spinothalamic tract
Pain is sent through the spinothalamic tract to the thalamus and through the spinomesencephalic tract to the periaqueductal grey (PAG)
2nd order neuron synapses with the 3rd order neuron and information is sent to the somatosensory cortex
3rd order neuron then sends information to the appropriate area of the homunculus of the primary somatosensory cortex
Rexed Lamina I
Located in the marginal layer
Respond to thermal and nociceptive input from Ad and C fibers
Rexed Lamina II
Located in the substantia gelatinosa
Respond to noxious stimuli and thermal stimuli from Ad and nonpetidergic C fibers
Rexed Lamina III and IV
Located in the nucleus proprius
In the deep dorsal horn, receive low threshold sensory information, not pain, mostly from AB fibers
Rexed Lamina V
Wide range of dynamic neurons
Second Order Neuron from Laminae II, IV, and V Path
Synapses with the reticular activating system (RAS) in the brainstem relaying information about:
Touch, vibration, and limb proprioception
RAS has projections to the medial thalamus and limbic system:
Mediates motor, autonomic, endocrine, and emotional response to pain
Second Order Neuron from Laminae I and V Path
Synapses with pons, medulla, midbrain, periaqueductal gray (PAG) and thalamus
Perception
Conscious awareness of pain
What are the components of the limbic system?
Cingulate gyrus
Amygdala
Hippocampus
Hypothalamus
Locus ceruleus
What does the cingulate gyrus deal with?
Behavior and emotion