Intermolecular Forces
1/25
Earn XP
Description and Tags
Exam 3 Prep
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Ion-Dipole Interactions
Are the strongest Intermolecular Force (IMF), but weaker than most covalent bonds
Ions With Multiple Charges
What is the condition for having a stronger ion-dipole interaction?
Permanent Dipoles
If molecules have this, they are attracted to each other.
Hydrogen Bonds (H-Bonds)
Are a subset of dipoles for molecules with N-H, O-H, And F-H bonds (stronger than most dipolar IMFs)
Higher Boiling Points
If you have a stronger IMFs, you have:
Hydrogen Bonds and Dipole-Dipole
Which IMFS are in effect only if the molecules are close to each other (short-range interactions)
Ammonia, Acetamide, Methanol, and Hydrogen Fluoride
What are some molecules that can form hydrogen-bonds with its own kind?
Hydrogens (H) bound to N, O, or F
How to tell how many H-bond donors a molecule has?
The number of lone pairs on N, O (or F-)
How to tell how many H-bond acceptor a molecule has?
Hydrogen Bond/ Molecule, Molecular Polarity, And Molar Mass
What affect the boiling point of a molecule?
Has Higher Heat Capacity Than Almost Any Other Material
Why is water a very special solvent?
High Surface Tension
H-Bonds of H2O molecules point away from air and thus have:
Salts And Electrolytes
Water is able to dissolve large amounts of:
Ion-Induced Dipole (ID) Interaction
When nearby ion polarize (distort) electron cloud of nonpolar group, inducing a temporary dipole.
Dipole-ID interaction
When nearby dipole polarize (distort) electron cloud of nonpolar group, inducing a temporary dipole.
ID-ID interaction
When a nonpolar group has a temporary dipole (due to random motion of its electrons) and induce a dipole in a nearby nonpolar group.
ID’s And Strong
___ are individually weak, but collectively can be ______
Ion-Dipole
Which Intermolecular Force is this model?

H Bond
Which Intermolecular Force is this model?
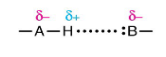
Dipole-dipole
Which Intermolecular Force is this model?
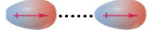
Ion-ID
Which Intermolecular Force is this model?
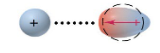
Dipole-ID
Which Intermolecular Force is this model?
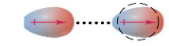
ID-ID
Which Intermolecular Force is this model?

Interact And Reacting
IMFs allow molecules to ______ without ______ (they can return to their original states)
Properties And Different Physical Forms
IMFs determine the ________ of bulk chemicals, and allow molecules to take on ____________________
ion > dipole > induced dipole (ID)
What is the rule for ranking intermolecular forces by strength?