Biochemistry Exam 2 University of Florida BCH4024 Prof Dan Purich, Biochemistry Exam 2 Dr. Zeile
1/904
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
905 Terms
needed for normal operation of metabolic pathways & main cellular functions
primary metabolites
functions of secondary metabolites?
warding off pathogens/predators; protecting against osmotic damage; treating various illnesses.
_____ are essential for cells to operate efficiently; pivotal role in metabolism; however, too little - not good, & too much - also not good.
Keto acids
what are secondary metabolites?
those organic compounds not needed for cell growth, development, or reproduction.
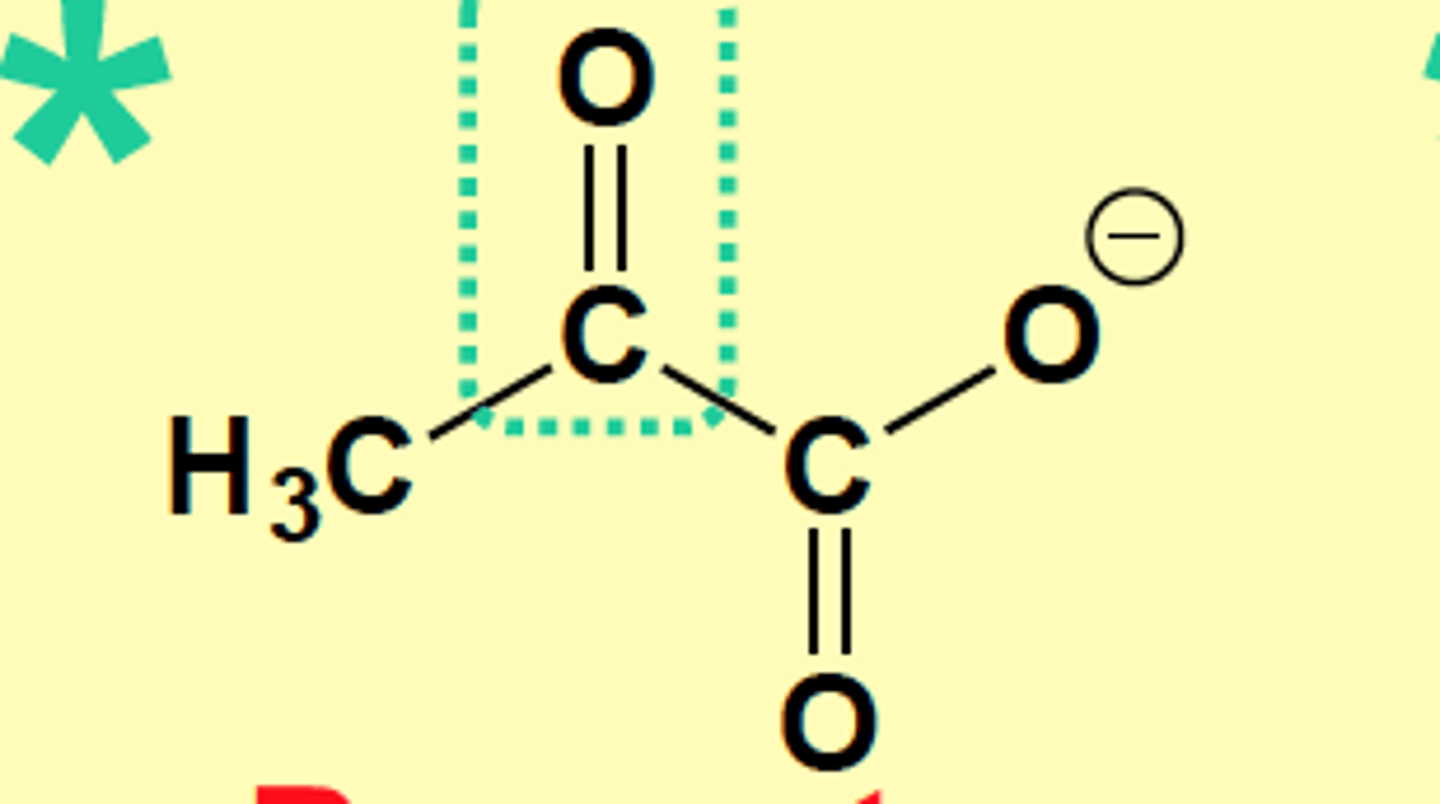
a-ketoacid of Alanine
Pyruvate
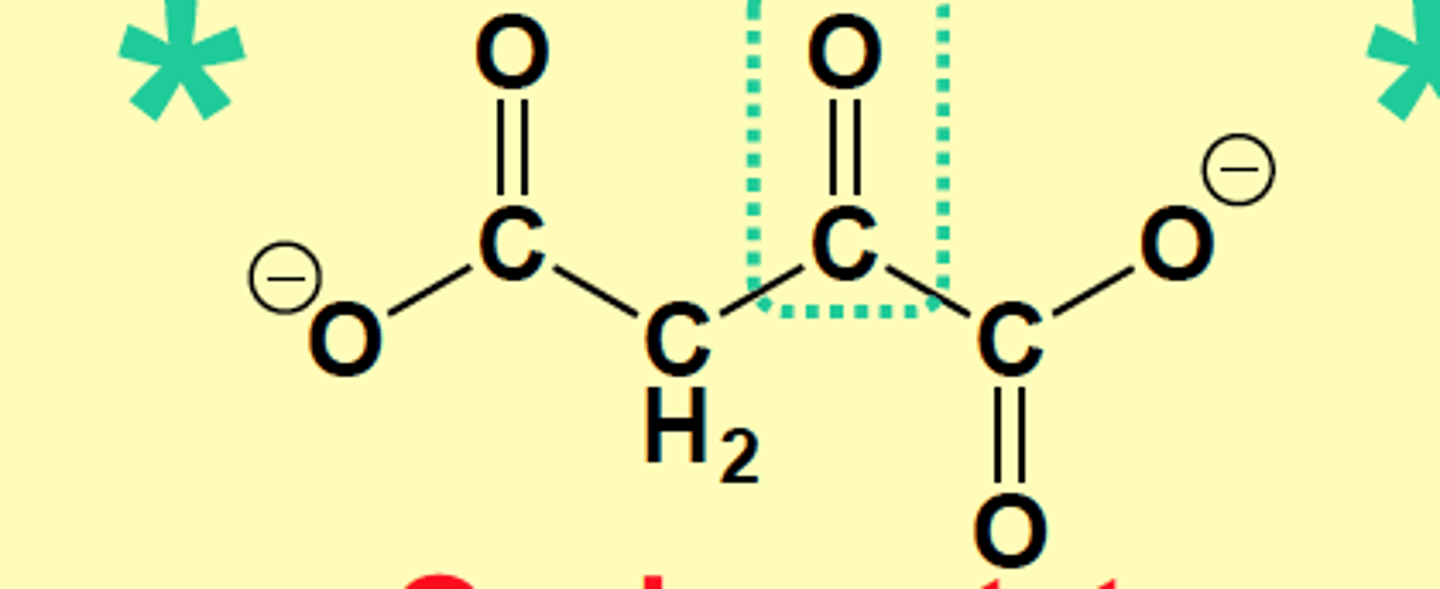
a-ketoacid of Aspartate, draw structure
Oxaloacetate
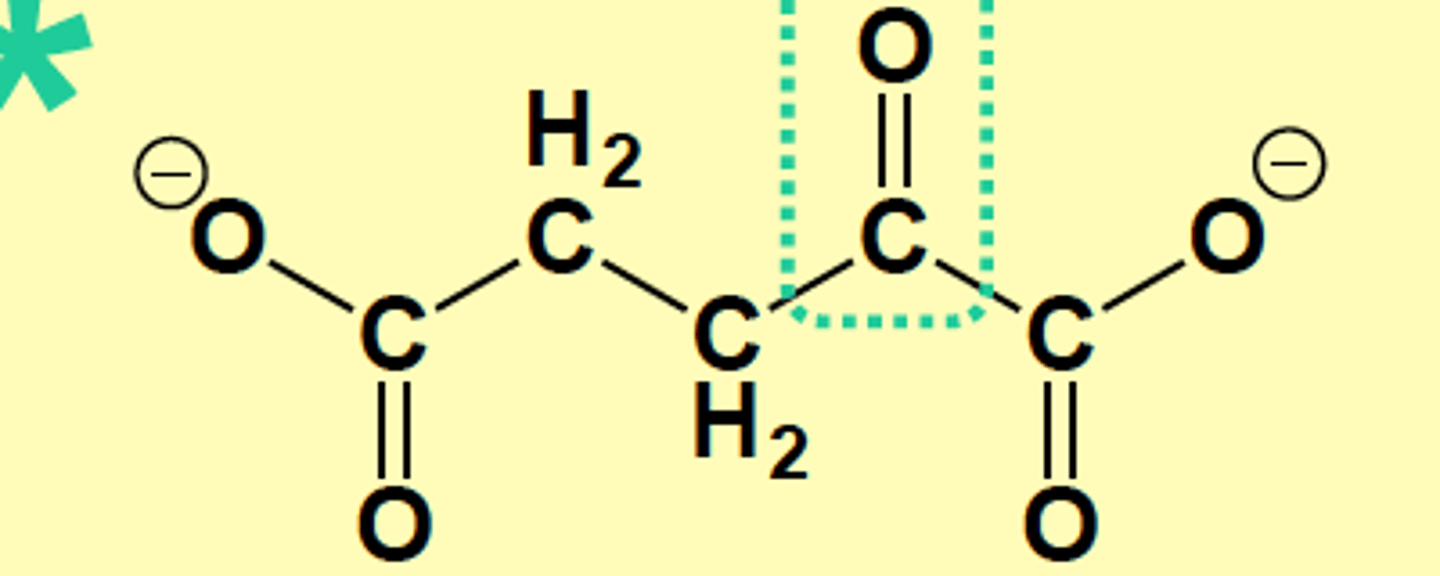
a-ketoacid of Glutamate, draw structure
a-Ketoglutarate
3 sources of amino acids
1) Digestion of proteins in foodstuffs
2) Intracellular proteolysis
2) de novoSynthesis of amino acids
Digestion of proteins in foodstuffs
Supply both essential and non-essential AAs
Intracellular proteolysis (4 points)
-Removes misfolded proteins
-Removes old and damaged proteins
-Regulates metabolism
-Controls cell-cycle transitions
de novo Synthesis of amino acids (5 points), and what does de novo mean?
-Provides amino acids needed for protein synthesis
-Adjusts amino acid pools in different tissues
-Adjusts energy metabolism by controlling levels of central pathway metabolites
-Allow cells to adapt to metabolic stress
-Needed to synthesizeneurotransmitters&nucleotides
synthesis of complex molecules from simple molecules
Nutritionally Essential Amino Acids:
Phe Val Trp Thr Ile Met His Leu Lys
PVT TIM HiLL
Conditionally Essential Amino Acids
Arg, Tyr, Cys
Arginine
essential for growth (childhood &pregnancy)
Tyrosine
essential, when Phe is inadequate
Cysteine
essential, when Metis inadequate
Diet must be varied toget adequate balance of ___________
amino acids
___________ have advantage by eating both plant & animal protein
Omnivores
Saliva
Mainly contains proteases from bacteria & white blood cells
Proteolysis
enzymatic cleavage of proteins, protein fragements, and amino acids + Di and Tri peptides
Intestines
-Contains neutral proteases-Chymotrypsin(cleaves on Carboxy side of aromatic residues) -Trypsin(cleaves on Carboxy side of lysine and arginine residues) -Carboxypeptidase(cleaves C-terminal AA) -Elastase(cleaves elastin, a highly elastic protein found in connective tissue)
At what point in the GI tract are proteins broken into fragments?
the intestines
Chymotrypsin
cleaves on Carboxy side of aromatic residues
Trypsin
cleaves on Carboxy side of lysine and arginine residues
Carboxypeptidase
cleaves C-terminal AA
Elastase
cleaves elastin, a highly elastic protein found in connective tissue
Digestion: Enzymatic Proteolysis (3 points)
1) Process begins with inactive proteases (zymogens)
2) Trypsinogen Activation
3) Pro-carboxypeptidase
Dietary proteins are _______ absorbed by healthy intestine
NOT- must be proteolyzed to amino acids, di-& tri-peptides
Process begins with inactive proteases (3 steps)
zymogens (name for inactive protease)
1) Zymogens are synthesized & stored in pancreas
2) Zymogens are secreted into small intestine
3) Only then are they converted to active catalysts
Trypsinogen Activation (4 steps)
1) Pancreas makes & stores trypsinogen in vesicles.
2) Secretory vesicles contain trypsin inhibitor (Prevents unwanted proteolysis of host cells)
3) Enterokinase, anectoprotease on intestinal mucosal wall, converts trypsinogen into trypsin
4) Trypsin activates chymotrypsinogen
Pro-carboxypeptidase
activated to carboxypeptidase
Removes AAs one-by-one from C-termini of food proteins
Zymogen Activity
1. Zymogen Synthesis, Processing & Transport (golgi complex releases for transport)
2. Vesicle Targeting & Release (zymogen granule enters the lumen)
How are zymogens activated?
by proteolytic cleavage
_________ enzymes are safer to store
inactive
Zymogen formation prevents _________ & __________
autophagy and apoptosis
Four major digestive proteases:
Name the zymogen, enzyme, and ph
Pepsinogen (S), Pepsin, Optimally active at pH 1-3
Chymotrypsinogen (PI), Chymotrypsin, Optimally activeat pH 7
Trypsinogen (PI), Trypsin, Optimally activeat pH 7
Procarboxypeptidase (PI), Carboxypeptidase, Optimally active at pH 7
S = Stomach PI = Pancreatic secretion into Intestine
Trypsin: 1st cleavage forms _________________
imperfect activation site
Chymotrypsin (cleaves out two dipeptides(14-15 & 147-148), 2nd Cleavage creates and "_____________" that stabilizes transition-state
oxyanion hole
active α-Chymotrypsin ( _______ covalently attached chains)
three
Pepsinogen activation is _________________
autocatalytic
Pepsinogen to pepsin
Slow Acid-Catalyzed activation by stomach[H+]
Once a little active pepsin forms, the latter catalyzes cleavage of many pepsinogen molecules to formmore catalytically active enzyme molecules
Pepsin operates at _____ pH by using its ________ carboxyl groups for catalysis ("Aspartic Proteinase")
low, aspartic acid
Intracellular Protein Turnover
Turnover rate depends on metabolic state .e.g., greater protein degradation, when nitrogen intake is low, because cells need amino acids to make vitally essential proteins
Two Major Pathways for Intracellular Protein Turnover
Lysosomal/Phagolysosomal Pathway
Ubiquitin-dependentPathway
Lysosomal/Phagolysosomal Pathway
Lysosome is an acidic compartment, where proteins undergo isoelectric expansion (partial unfolding).
Low pH makes them more susceptible to proteolysis
Ubiquitin-dependent Pathway
Ubiquitin is a 8.5-kDa protein that is enzymatically joined to poorly folded proteins.
Ubiquitinated proteins are degraded in proteasomes, barrel-like macromolecular protease complexes
AA & Na+are transported togetherin same direction....called a ____________
symporter
Transport driven by _____________: ______ Na+ in intestinal lumen _____ Na+in brush border cell
trans-membrane ion gradient. High ,Low
Cell transporters use ______________ to drive transport(drives Na+out &K+in)
ATP hydrolysis
Amino acid transporters maintain _______________
chemiosmotic Na+concentration gradient
Nitrogen Balance
Relates Nitrogen intake to Nitrogen excretion
True nitrogen balance:
Intake = Excretion
Positive nitrogen balance:
Intake > Excretion
Negative nitrogen balance:
Excretion > Intake
Positive Nitrogen balanceis required for: (4)
-growth in childhood
-growth in pregnancy
-healing of wounds
-convalescence
Negative Nitrogen balance occurs during: (3)
-starvation
-malnutrition
-disease (burns, trauma, surgery)
Marasmus
Grossly underweight
No body fat
Muscle wasting
Old man's face
Normal hair
Lethargic
Malnutrition associated with ____________________________________, with little/no edema.
extensive tissue and muscle wasting
Features of marasmus (2)
loose folds of skinhanging over buttocks
Severe deficiency of nearly all nutrients, especially protein, carbohydrates, and lipids
"_______________" resulting from inadequate intake of Protein & Calories (marasmus)
Protein-Energy Malfunction
Kwashiorkor translation
Translation: "sickness last baby getsthe new baby arrives"
Kwashiorkor:
Acute childhood protein malnutrition
How does Kwashiorkor differ from marasmus?
Inadequate protein intake, BUT otherwise adequate caloric intake
Characteristics of Kwashiorkor (3)
Irritability (neurotransmitter deficit)
Enlarged liver(fatty infiltrates)
Abdominal edema-caused by hypoalbuminemia
Transaminases Use Vitamin _____ Coenzymes
B6
pyridoxal phosphate (PLP)
The active coenzyme form of vitamin B6
Has an aldehyde
Pyridoxamine phosphate (PMP)
Has CH2-NH2 group
Transaminases catalyze two half-reactions:
Amino Acid1+ Enz-PLPα>>>>>a-Keto Acid1+ Enz-PMP
α-Keto Acid2+ Enz-PMP>>>>>>Amino Acid2+ Enz-PLP
Sum: amino acid1+ α-Keto Acid2>>>>amino acid2+ α-Keto Acid1
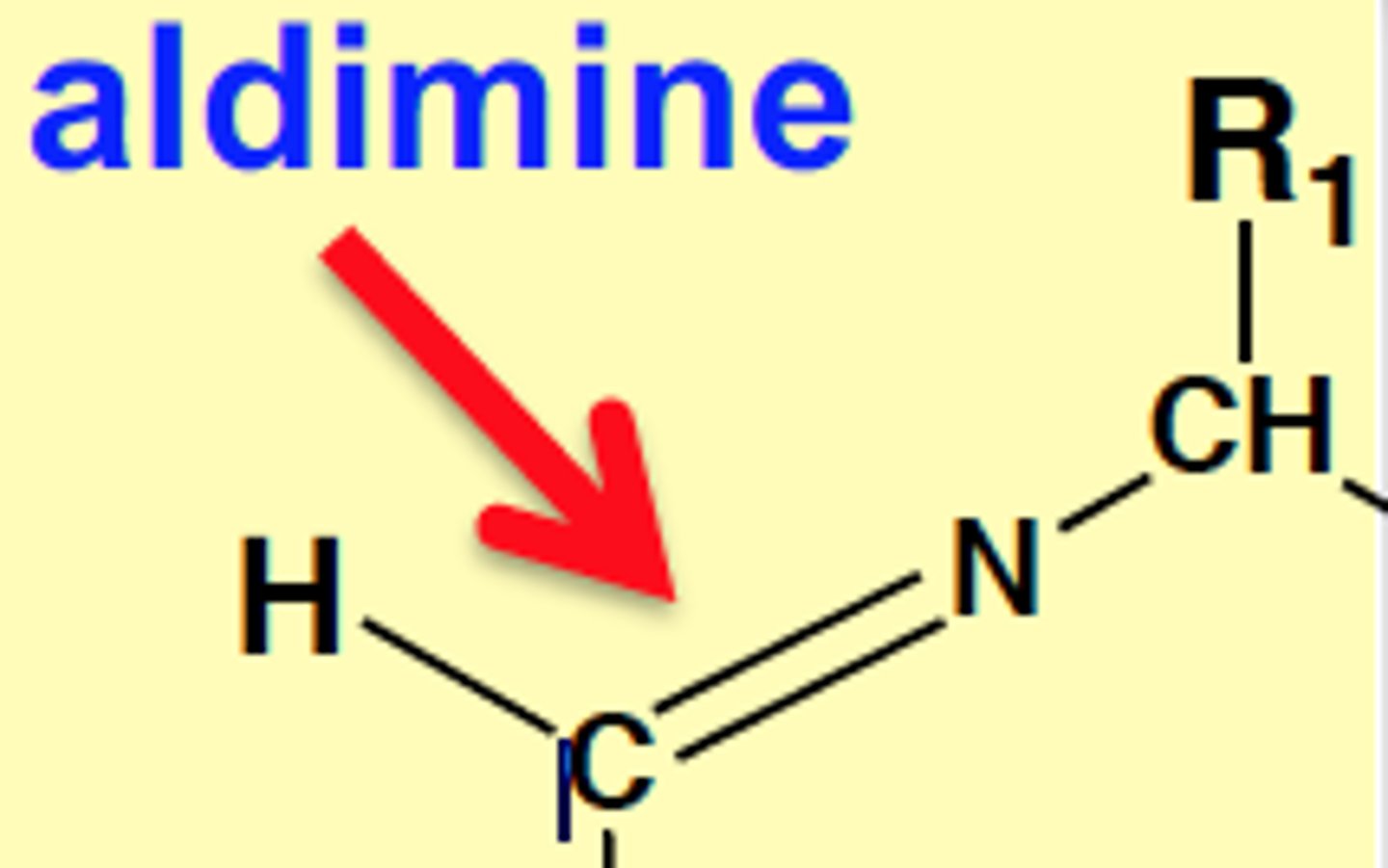
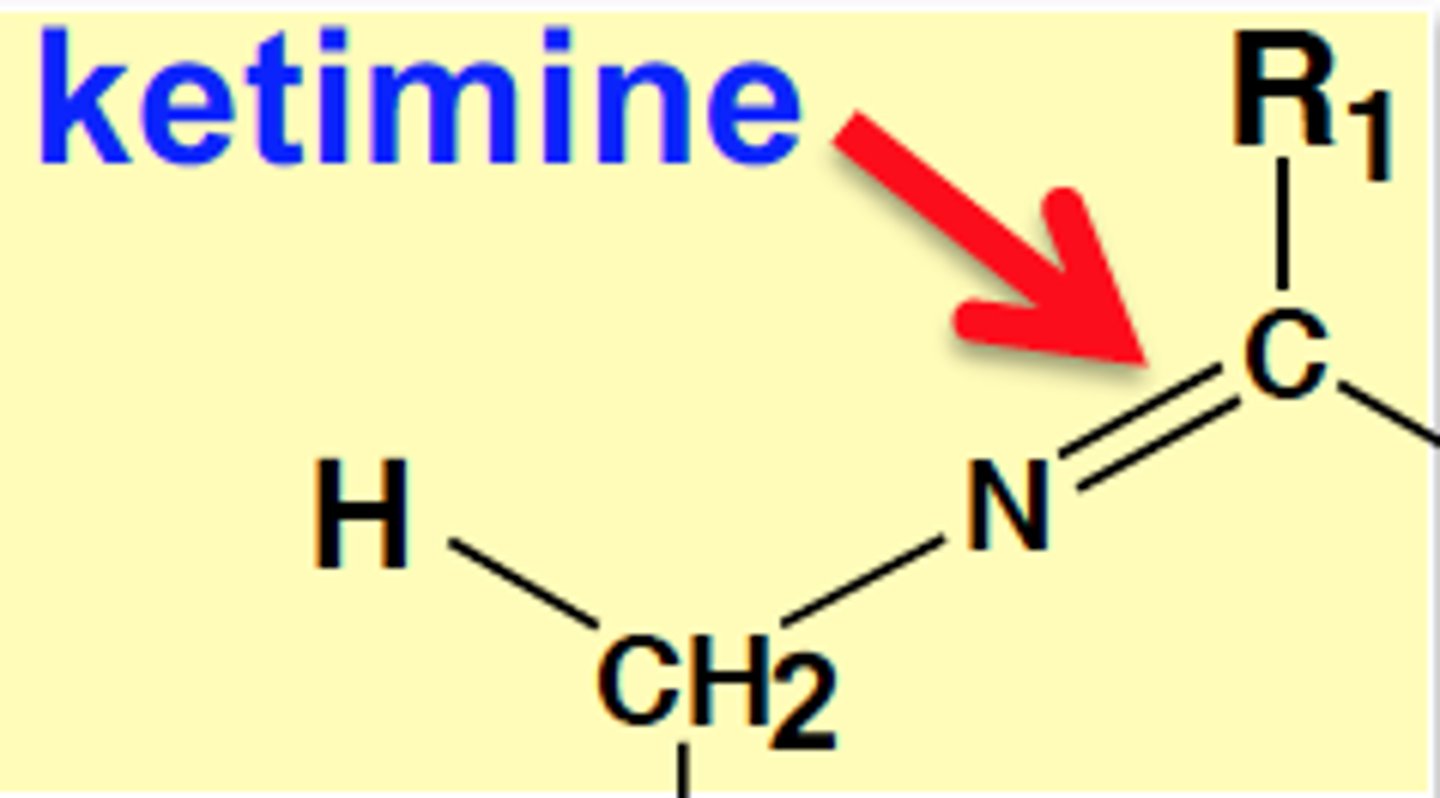
Mechanism of Enzyme-bound Reactions (2 half-reactions)
1st Half-reaction:
-Aldimine forms
-Converts to ketimine
-Hydrolyzes to ketoacid
2nd Half-reaction
is reverse of 1st half-reaction, using R2-KA to make R2-AA
Aldimine
H-C=N-CH-R1
Ketimine
H2-C-N=C-R1
_____ deficiency impairs many AA reactions and formation of some AA-derived neurotransmitters
B6
________ bunds extremely tightly in transamination
PLP
Transamination reactions are fully ________
reversible
Transamination reactions Keq
Keq= 1 (same bonding in substrates and products).
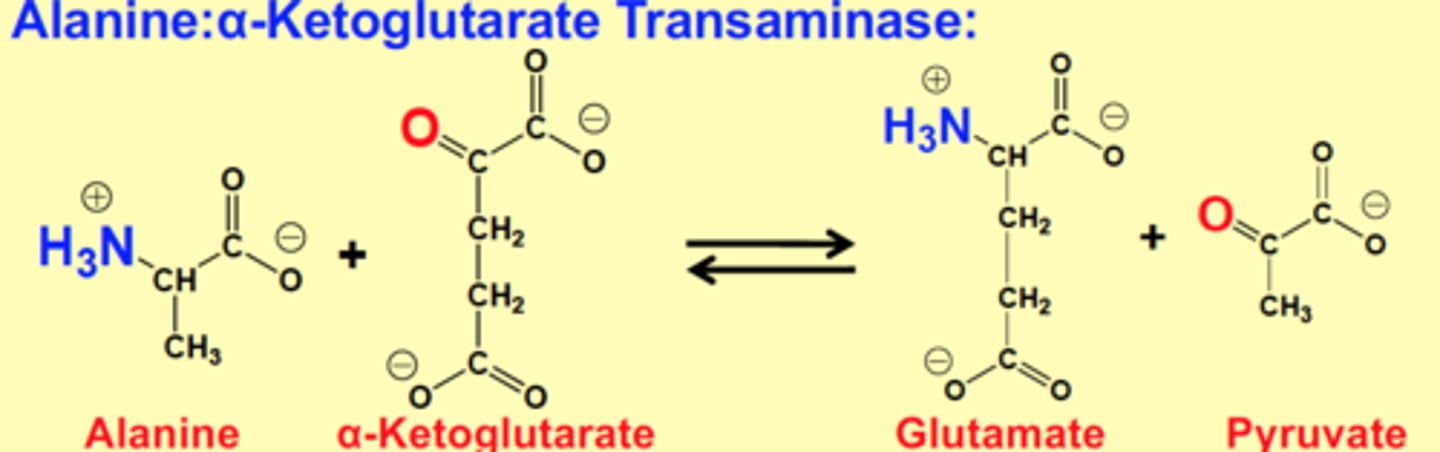
Alanine:α-Ketoglutarate Transaminase
Alanine + α-Ketoglutarate > Glutamate + Pyruvate
NH3 from alanine and carboxyl from α-Ketoglutarate is switched to form new products
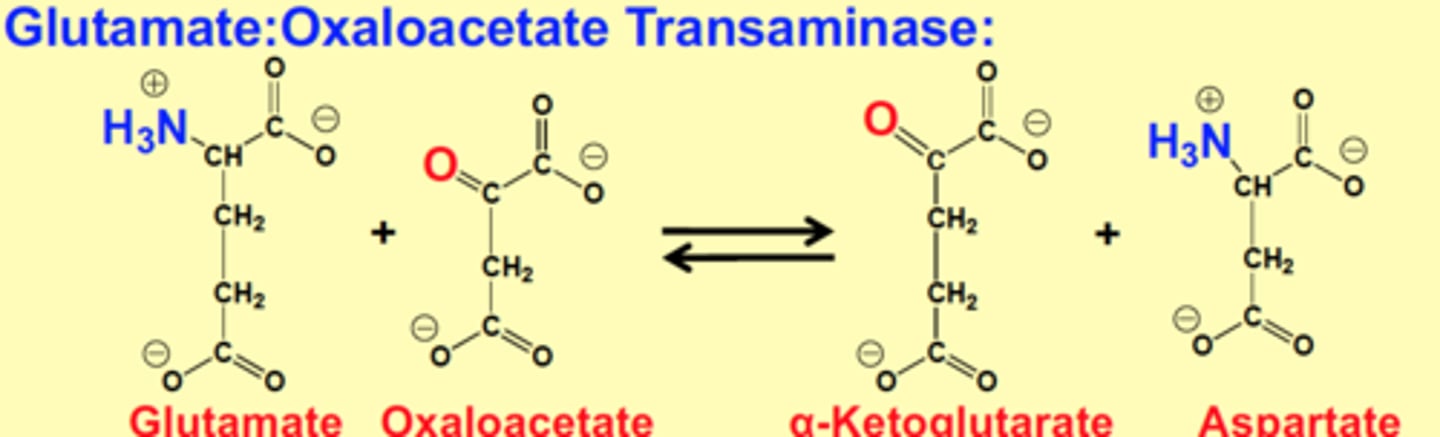
Glutamate:Oxaloacetate Transaminase
Glutamate + Oxaloacetate > α-Ketoglutarate + Aspartate
NH3 from glutamate and carboxyl from oxaloacetate is switched to form new products
Transamination is ________
selective
All but _____ amino acidsare transaminated
4
_________ & __________ are secondary amines, and, as such, they lack primary amino groups needed to undergo transamination
proline and hydroxyproline
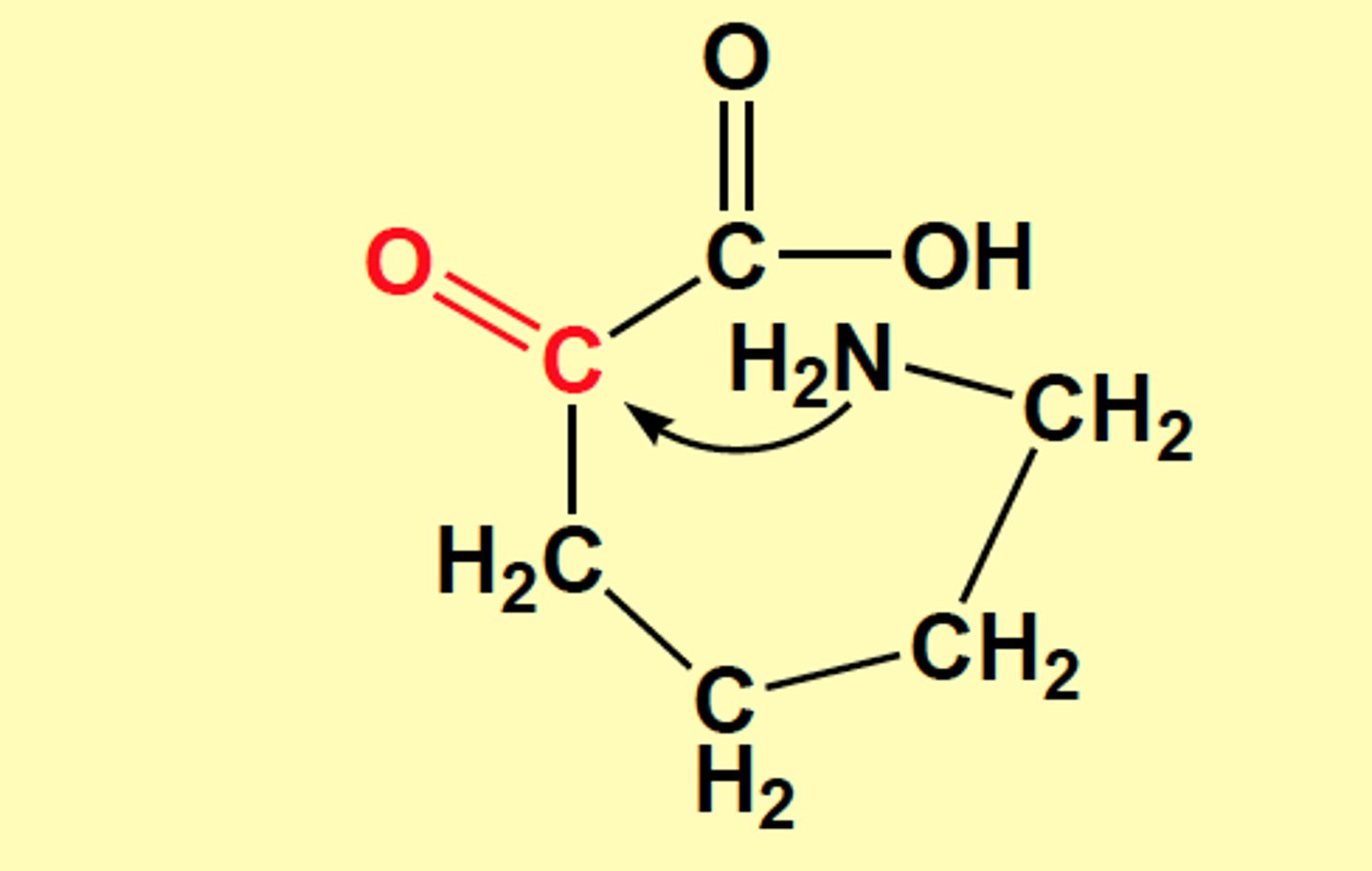
If ______ were to undergo transamination, the keto acid would cyclize to form a toxic nonmetabolite
lysine
N of NH2 attacks carbonyl
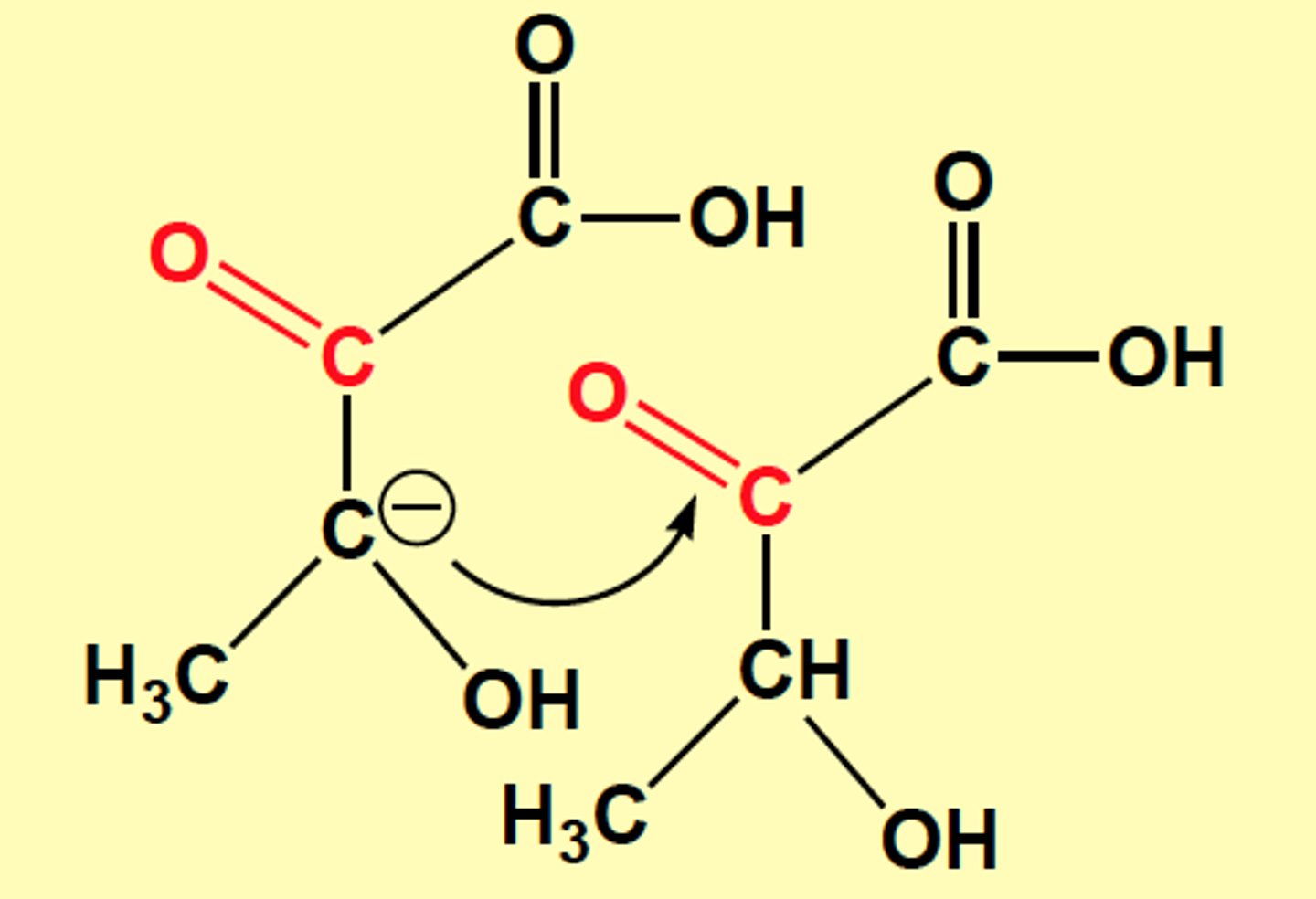
If ______ were to undergo transamination, the keto acid would dimerize into a toxic nonmetabolite
threonine
Transaminases ________ bind lysine or threonine
CANNOT
3 possible routes for deamination
oxidative, hydrolytic, eliminative
Glutamate Dehydrogenase (2) and reaction
Major route for oxidative deamination
Regenerates the amino acceptor (α-ketoglutarate) and provides ammonia, either for re-utilization ordisposal as urea
glutamate + NAD+ + H2O > α-ketoglutarate + NADH + NH3
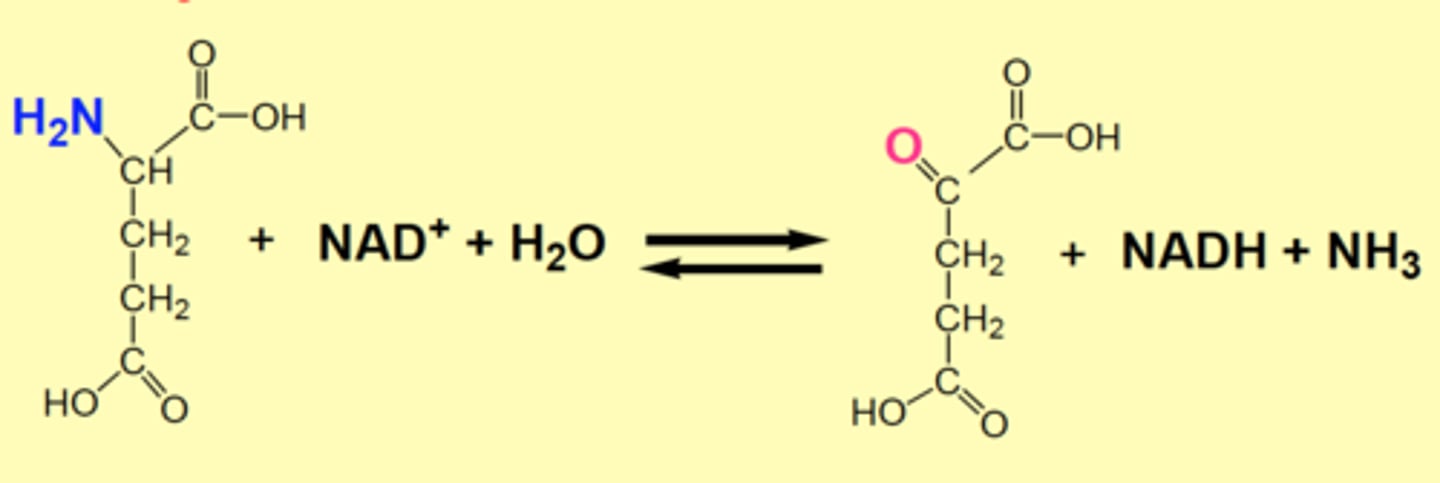
GDH is located in the
mitochondrial matrix
Draw the structure of α-ketoglutarate
Slide 29
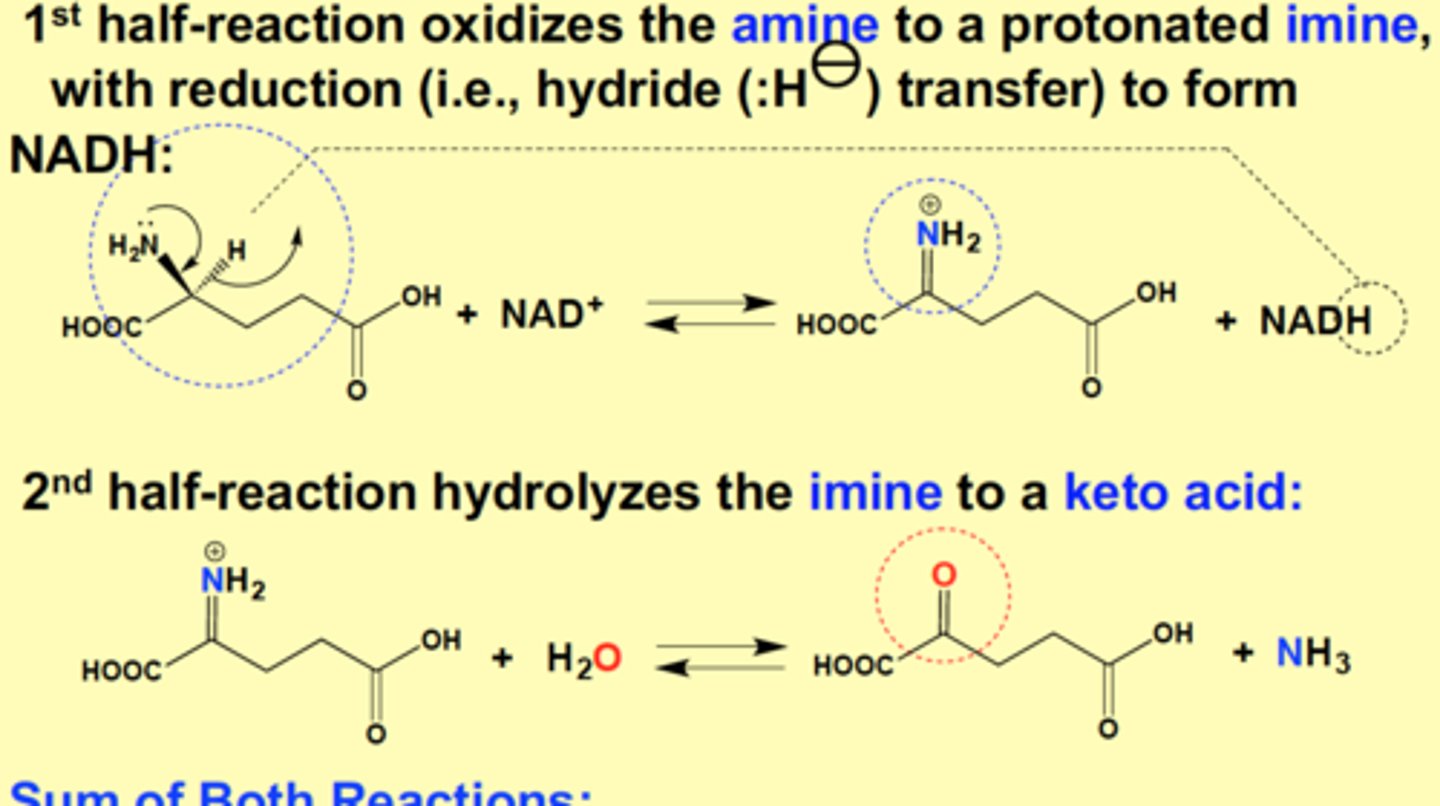
Glutamate Dehydrogenase Mechanism
1st half-reaction oxidizes the amine to a protonated imine, with reduction (hydride transfer) to form NADH
2nd half-reaction hydrolyzes the imine to a keto acid
Sum of Both Reactions:
Glu + NAD+ + imine + H2O > aKG + Imine + NADH + NH3
Glu + NAD+ + H2O >> aKG + NADH + NH3
NAD+ > NADH
Gains H- (reduced)
NADH > NAD+
Lose H- (oxidation)
By storing electrons, ________ & _______ allow cells to manage chemical energy. Also, they are natures batteries
NADH & NADPH
______________ yields energy needed to make 3 mol ATP (Process called:__________________)
NADH oxidation yields energy needed to make 3 mol ATP (Process called: Oxidative Phosphorylation)
Structural differences between NAD+ and NADP+
NAD+ is oxidized
NADP+ has another phosphate group
The part that undergoes redox is a Nitrogen benzene with amide attached (not proper name)
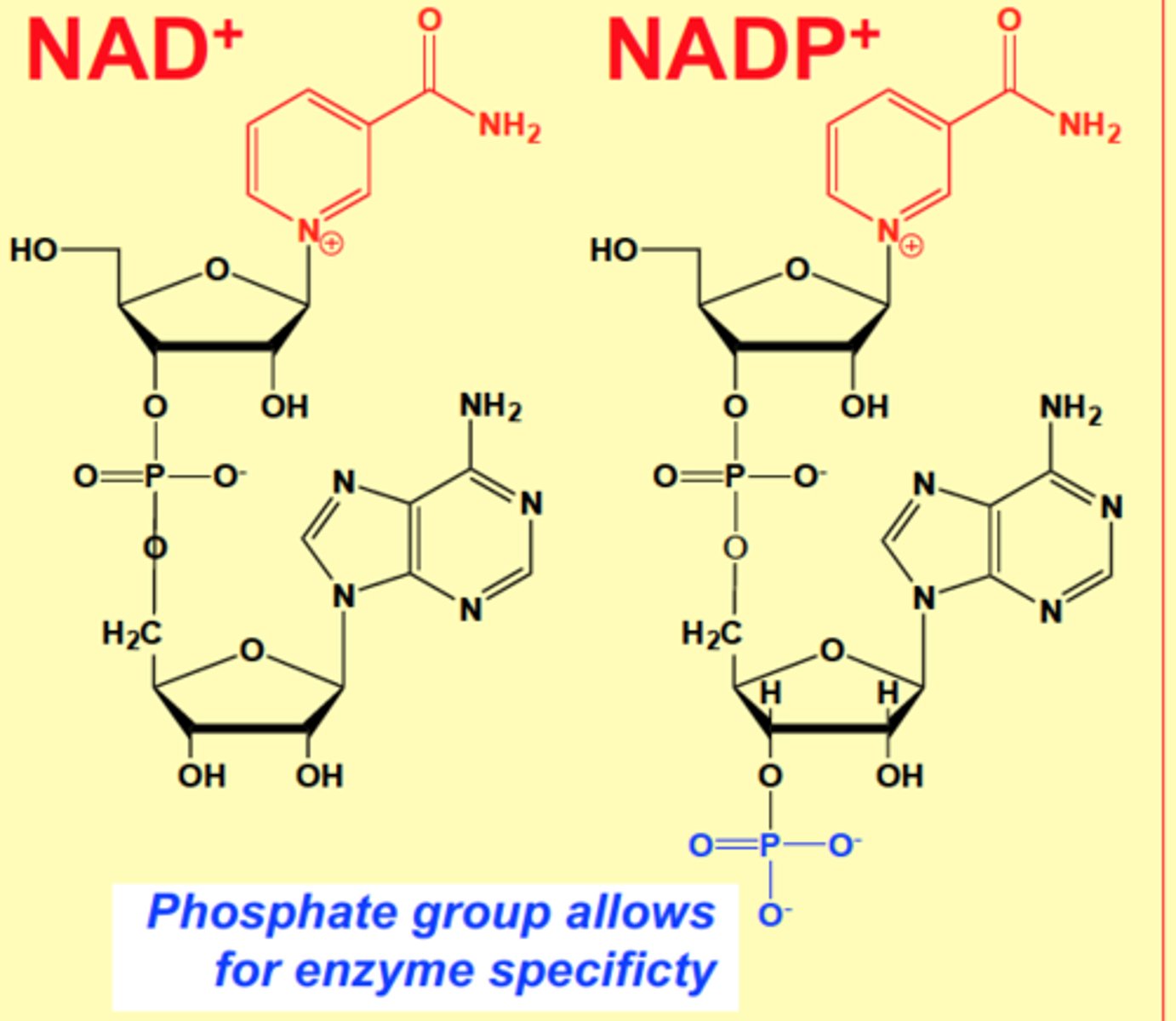
GDH uses _____ or _____
NAD+or NADPH
______ is used in the oxidative deamination reaction
NAD+ (i.e.formation of ammonia and α-ketoglutarate)
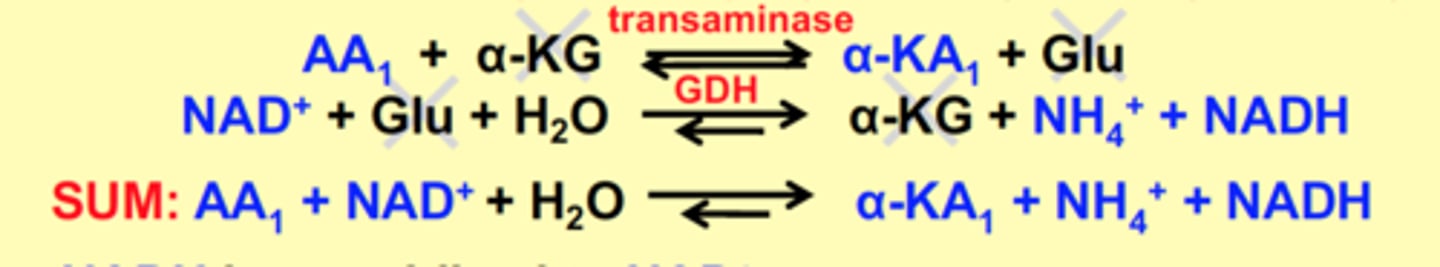
The coupling of _______ with __________ allows foroxidative degradation of 14 other amino acids.
What are the exceptions?
GDH, transaminases
Exceptions: Pro, Hyp, Thr, Lys, & His
NADH
is re-oxidized to NAD+ in Oxidative Phosphorylation
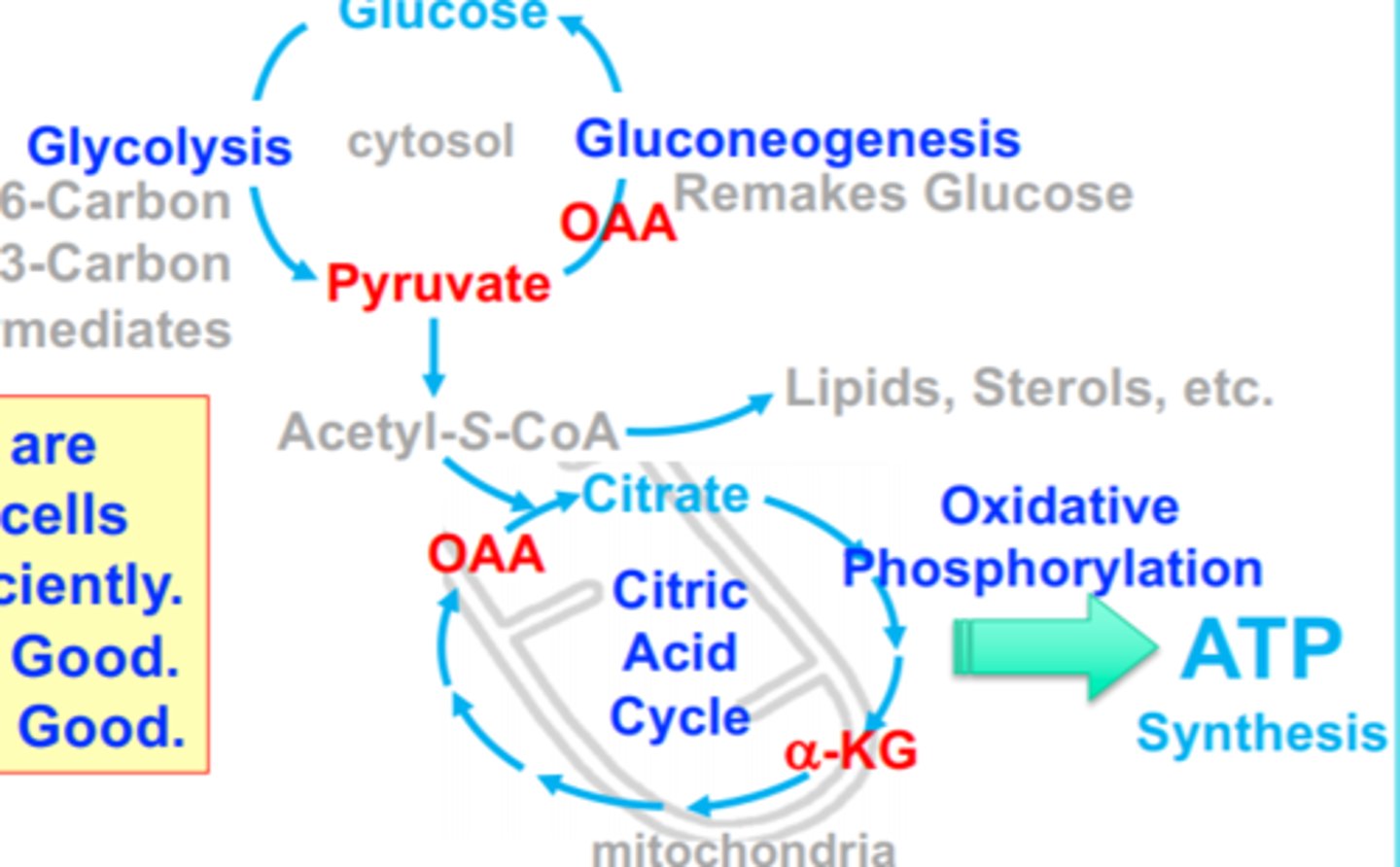
α-KA
enters Tricaroxylic Acid Cycle (Citric Acid Cycle)
Excess NH4
+
enters the urea cycle
Operating in the opposite direction ,_____ drives reductive amination to make glutamate
How?
NADPH
hydrolysis? (check)