internal energy
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
What happens to the energy when a solid turns to a liquid then gas
Kinetic and potential energy increase.
Why does the kinetic and potential energy increase when a solid changes states
Particles more faster
Potential energy increases as the positions of the particles relative to eachother increase.
Some of the inter molecular forces are overcome
What is intermolecular forces?
The attractive forces that exist between molecules,
What's internal energy?
The sum of the randomly distributed kinetic and potential energies of its particles
What's the difference between thermal and internal energy?
Thermal energy is sum of randomly distributed kinetic energy whereas internal is sum of kinetic and potential
When does internal energy increase?
When energy is transferred to a system by heating
When does internal energy decrease?
When energy istransfered from a system by heating
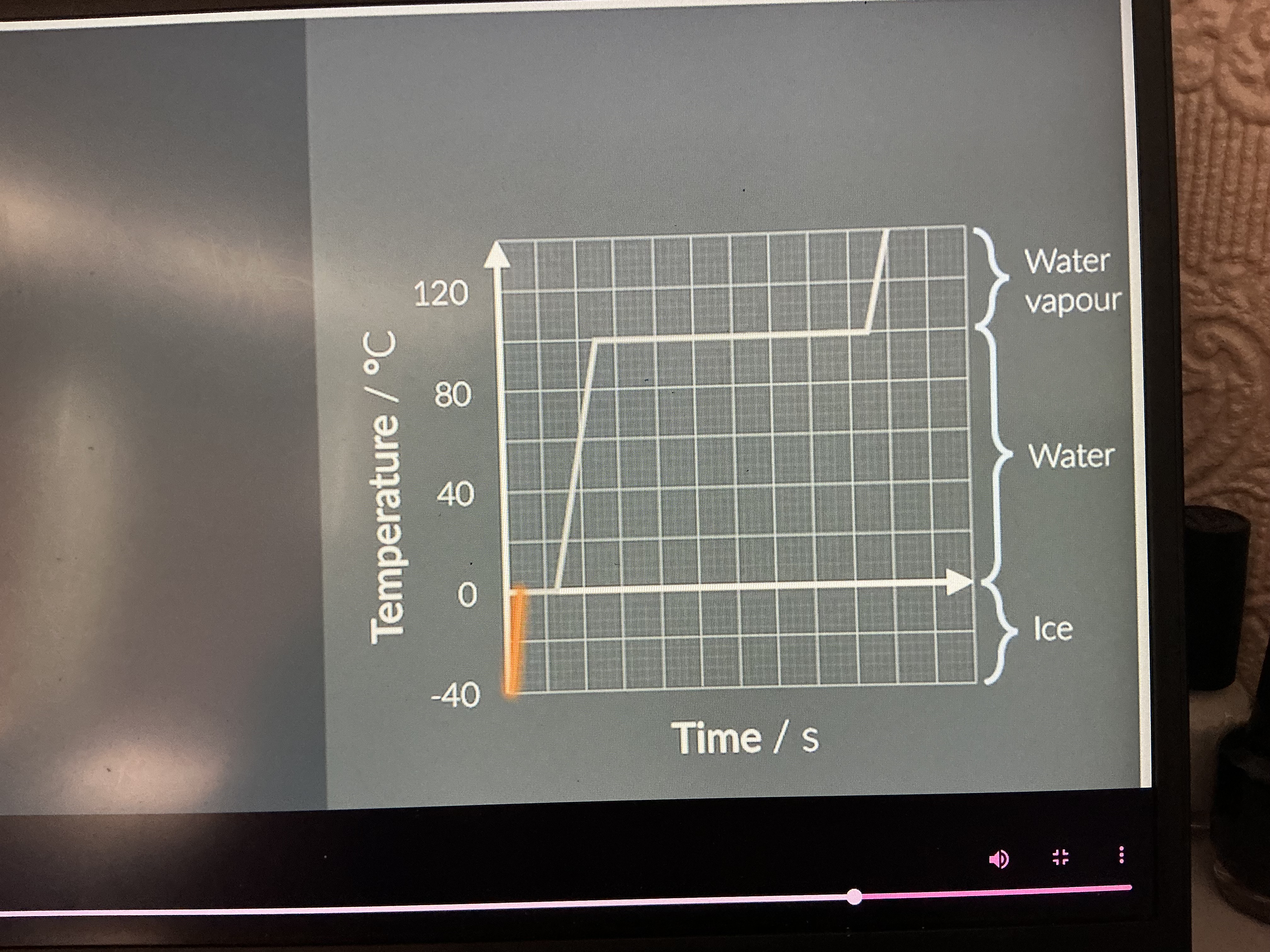
What's happening?
Large increase in average kinetic energy and small increase in potential energy
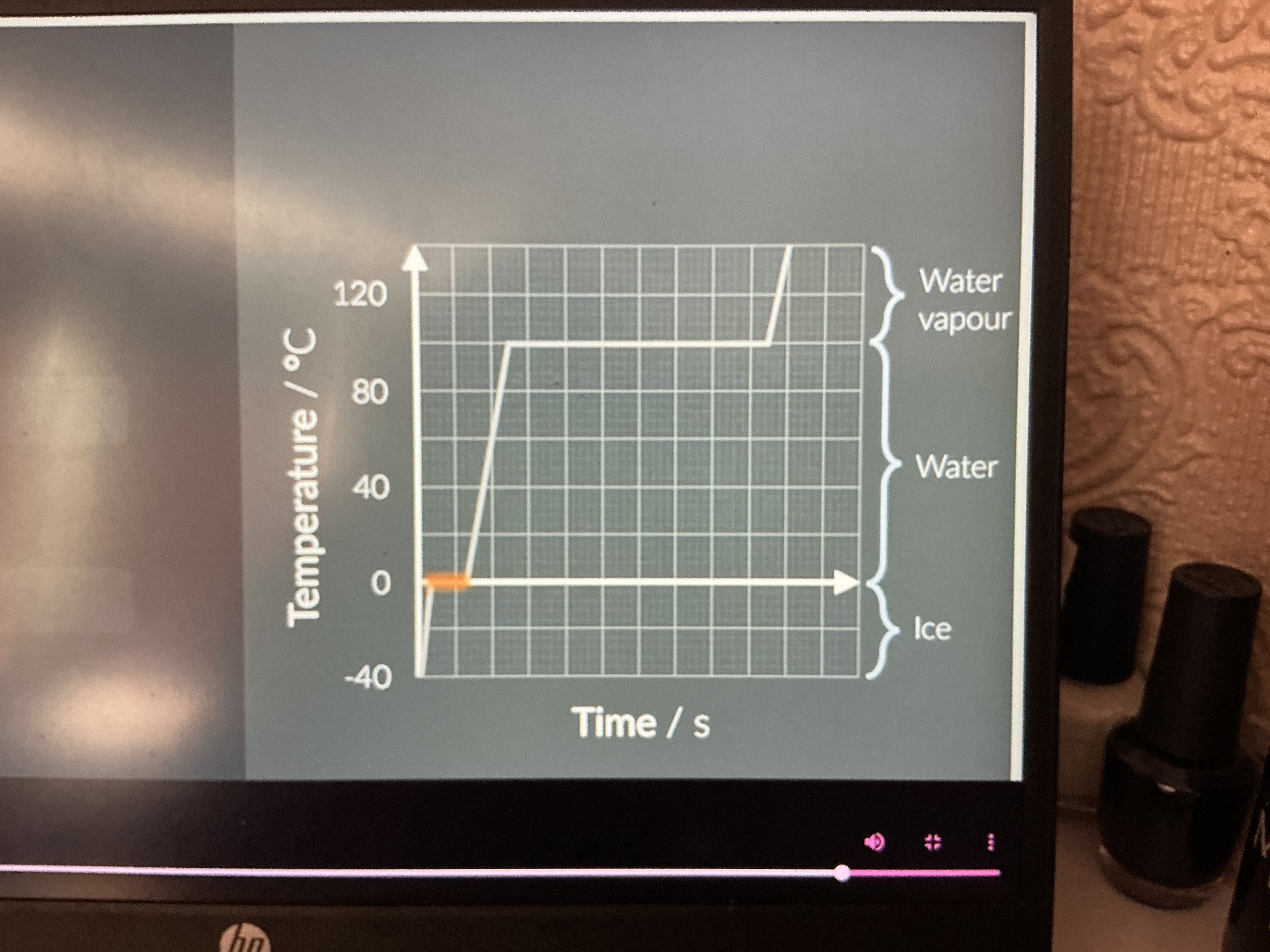
What's happening here?
The particles are undergoing a change of state where only the potential energy increases
Why does the temperature stay the same when a system undergoes change of state.
During a change of state the energy suppled to the system is used to overcome the intermolecular forces between the particles rather than to increase their kinetic energy
What happens to the internal energy of a system when work is done on a system?
Increase → rubber band stretched
.What happens to the internal energy of a system when work is done by a system?
Decreases → air from tyre released
What causes internal energy to increase?
Heating and doing work
How do you work out the change in internal energy?
Energy transfer due to heating minus the workdone on the system
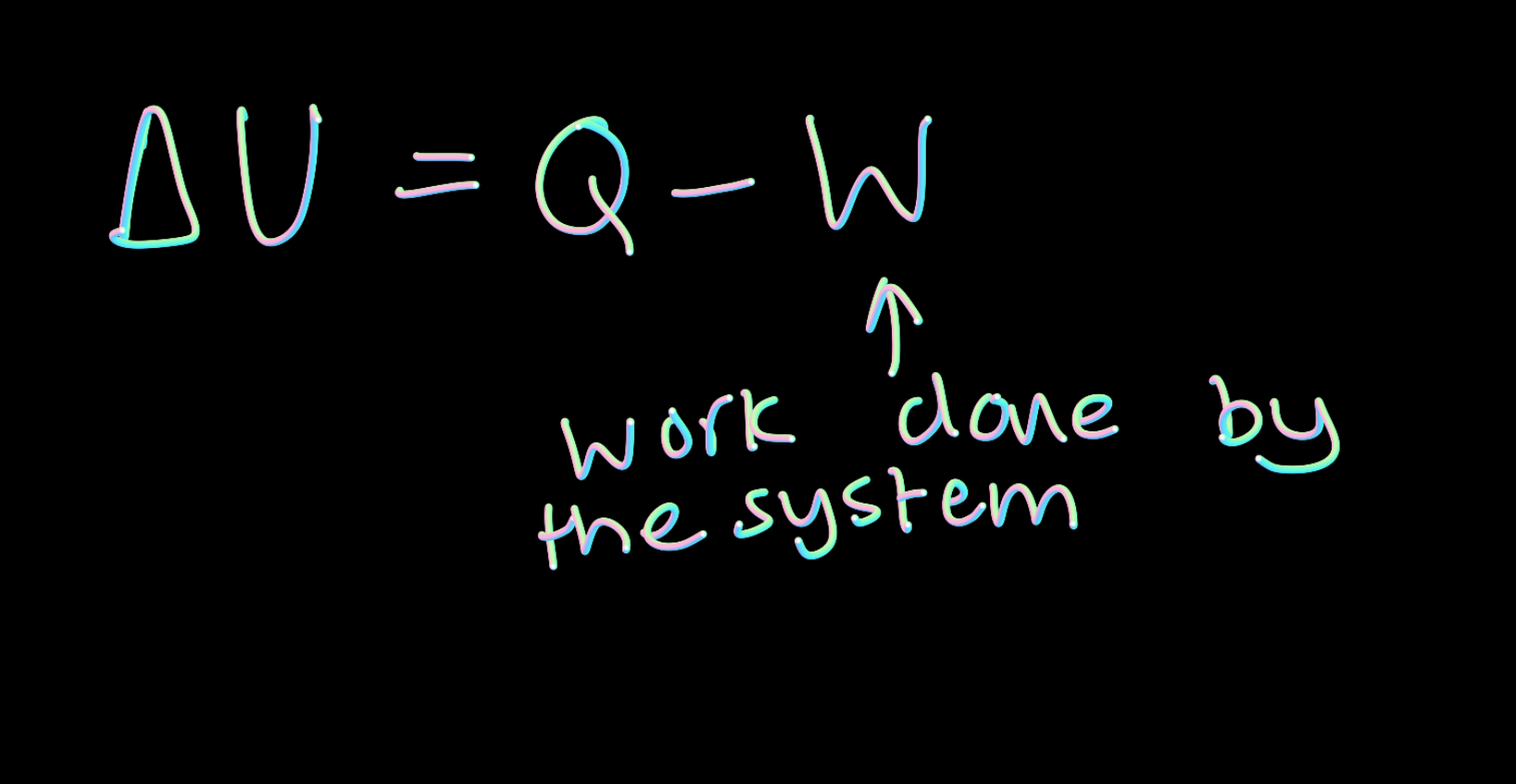
What are the conditions for the internal energy of a system to remain constant.
No energy is transferred by heating or by work
The energy transferred to the system by heating is equal to the work done by the system
The energy transferred from the system by heating is to the work done on the system
When is the average kinetic energy greater than the average potential energy?
When the temperature of the object increases because the particles kinetic energy is increasing
What's happening to temperature during change of state
Stays the same
What changes internal energy?
Heating and work