Comprehensive Cancer Genetics and Embryology: Key Concepts and Mechanisms
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
What is cancer?
A complex heterogeneous disease that occurs when cells breach anti-cancer defense mechanisms.
What are the types of tumors?
Benign (non-invasive but proliferative), Primary tumor (first tumor cells), Malignant (cells that invade surrounding cells), and Metastatic (cells that spread to distant organs via blood/lymph).
What is leukemia?
A cancer characterized by the overproduction of immature white blood cells from myeloid or lymphoid progenitor cells, which can be caused by genetic factors.
What is lymphoma?
A cancer that originates from cells in the lymph nodes.
What are the origins of cancer?
Cancer originates from genetic/epigenetic changes, environmental exposures (like chemicals), genetic factors, and infectious diseases.
What role do genetic lesions play in cancer?
Genetic lesions can lead to mutations that contribute to cancer development.
What is an epigenetic change?
An alteration in gene expression caused by mechanisms such as methylation, which can silence genes.
What are genetic drivers in cancer?
Genes that are mutated in cancer cells and directly contribute to cancer development.
What is the Cancer Gene Census?
A catalog of gene mutations that directly cause cancer.
What are common genetic drivers of cancer?
TP53 mutations and KRAS mutations.
What are passenger mutations?
Mutations found in cancer cells that do not directly promote cancer development.
What are the hallmarks of cancer according to the new model?
1. Sustaining proliferative signaling, 2. Evading growth suppressors, 3. Resisting cell death, 4. Enabling replicative immortality, 5. Inducing angiogenesis, 6. Activating invasion and metastasis, 7. Cellular metabolism.
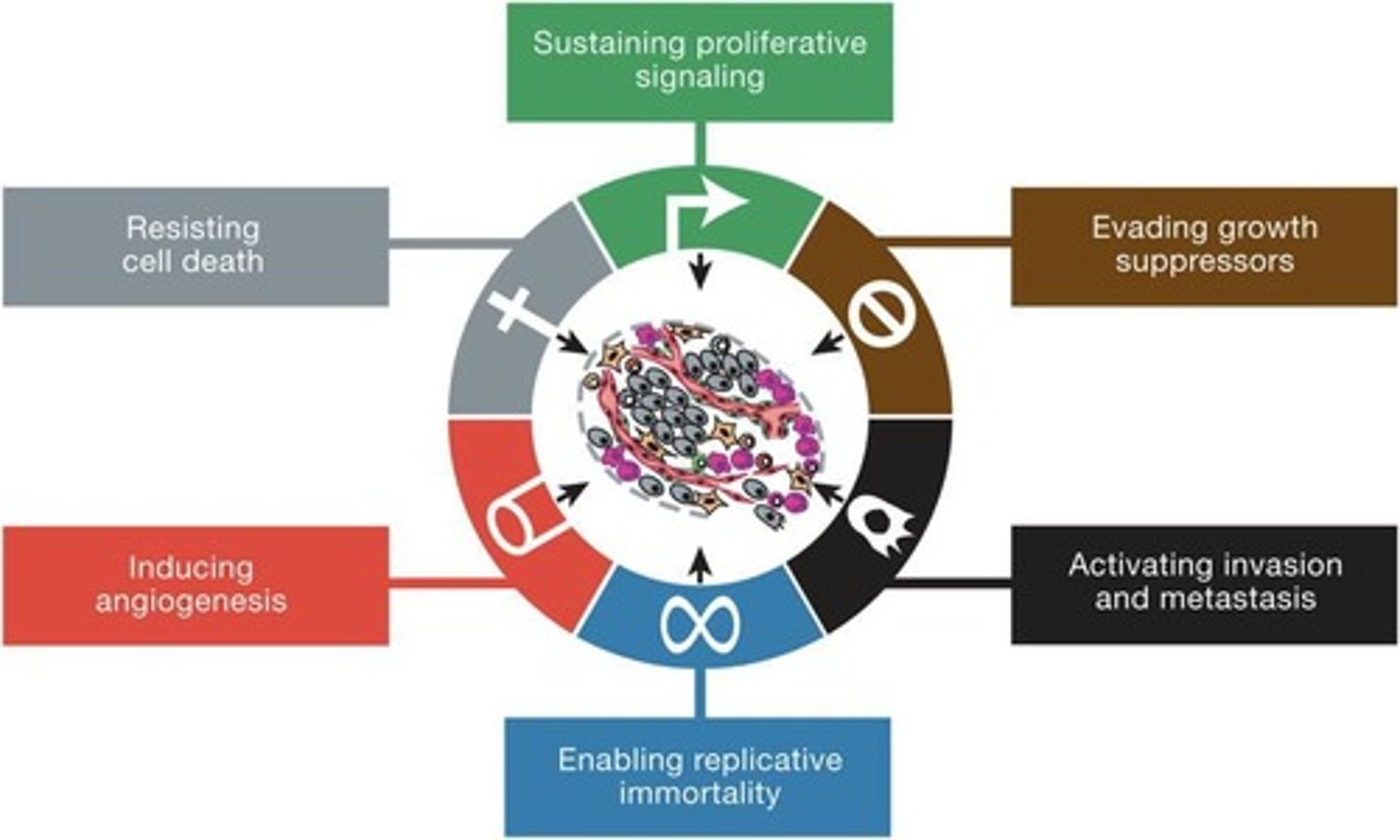
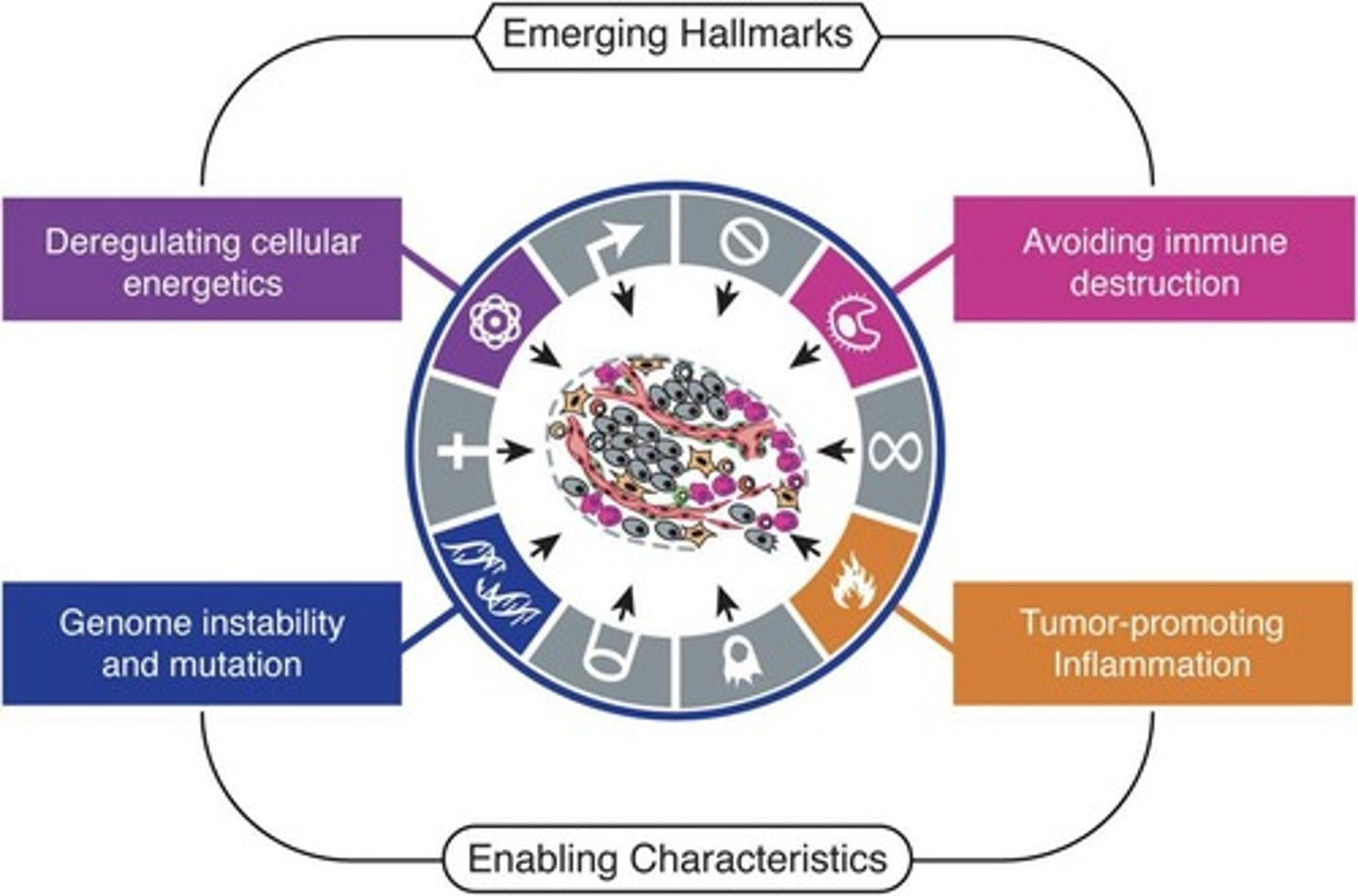
What are the enabling characteristics of immune evasion in cancer?
Genome instability and inflammation.
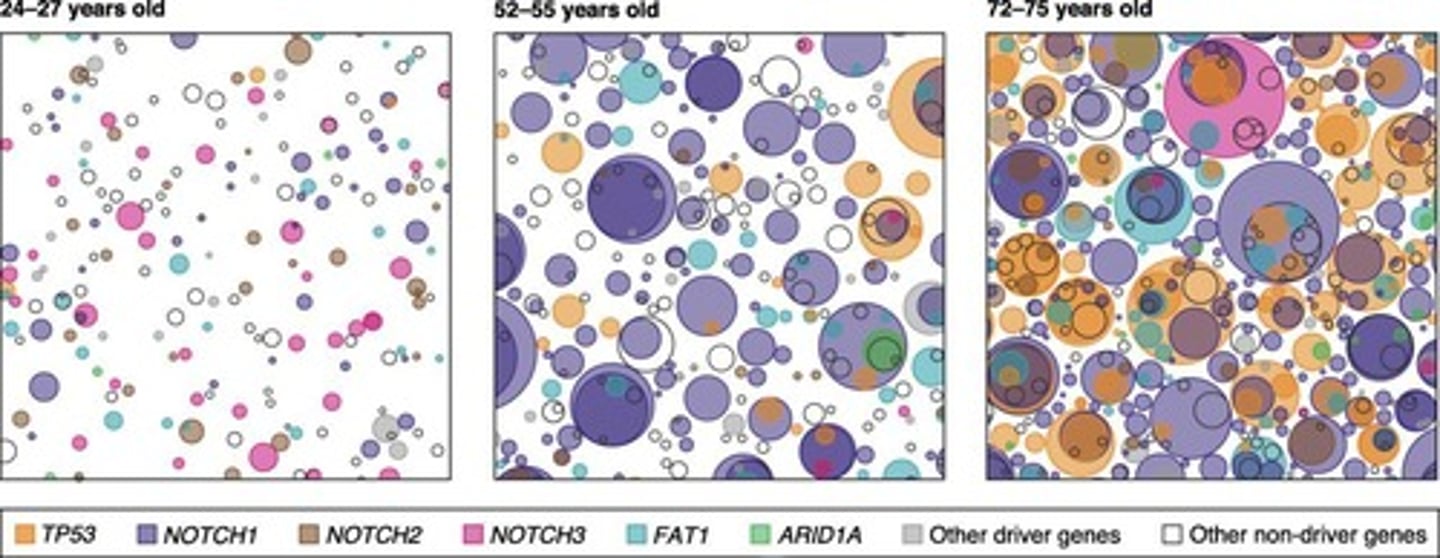
What is the main intrinsic barrier to tumorigenesis?
p53, which signals for DNA repair or apoptosis if damage cannot be repaired.
How does mutated p53 contribute to cancer formation?
It allows continued cell cycle progression and evasion of apoptosis.
What happens to cancer risk as age increases?
Cancer risk increases with age due to the accumulation of somatic mutations.
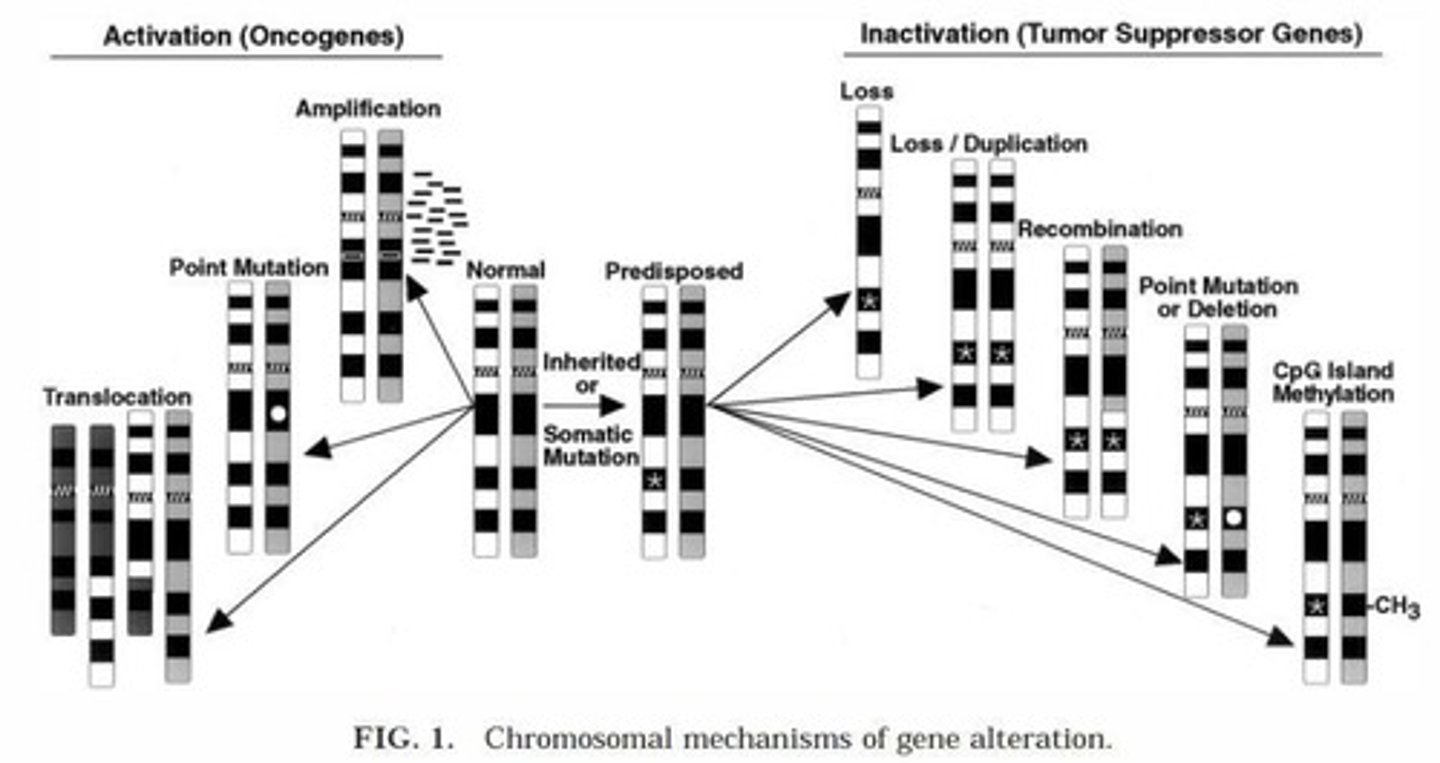
What are oncogenes?
Genes that, when mutated, promote cell growth and proliferation, often through gain-of-function mutations.
What is the role of MYC in cancer?
MYC is a key oncogene that orchestrates various hallmarks of cancer.
What are tumor suppressor genes?
Genes that inhibit cell growth; both alleles must be inactivated for loss of function.
What is gene silencing in cancer?
It is caused by methylation of CpG islands, leading to the inactivation of tumor suppressor genes.
What is the significance of TP53 and RB in tumor suppression?
They are key proteins that control tumor suppressor pathways.
What is the impact of ionizing radiation on DNA?
It causes DNA breakage, which is recognized by ATM, leading to p53 activation.
What is Li-Fraumeni Syndrome?
A genetic condition caused by mutations in the TP53 gene, associated with a high risk of various cancers.
What is the embryonic period in pregnancy?
The first 8 weeks after fertilization, during which the embryo is most susceptible to teratogens.
What occurs during fertilization?
The union of a secondary oocyte and spermatozoa, leading to the formation of a zygote.
What is the process of the acrosome reaction?
The process by which enzymes are released from the sperm to penetrate the zona pellucida.
What is the role of calcium during fertilization?
It blocks polyspermy and resumes meiosis in the egg.
What is the blastocyst?
A structure formed from the morula that contains an inner cell mass and trophoblast, crucial for implantation.
What are the two layers formed in the bilaminar embryonic disc?
Hypoblast and epiblast.
What is the primitive streak?
A structure that defines the major body axes and is crucial for gastrulation.
What is gastrulation?
The process where the bilaminar embryonic disc becomes trilaminar, forming the endoderm, mesoderm, and ectoderm.
What is the role of the trophoblast during implantation?
It attaches to the endometrium and secretes enzymes to facilitate burrowing of the blastocyst.
What is the significance of the zona pellucida?
It must be eroded for the blastocyst to implant into the uterine wall.
What is Familial Adenomatous Polyposis (FAP)?
A genetic condition caused by mutations in the APC gene, leading to colorectal cancer.
What is Lynch Syndrome?
A hereditary condition associated with mutations in DNA mismatch repair genes, leading to increased risk of colon cancer.
What are common variants in cancer genetics?
Genetic variations that have low penetrance and are associated with a low chance of developing cancer.
What are rare genetic variants in cancer genetics?
Genetic variations that have high penetrance and are associated with a significant risk of developing cancer.
What is gene silencing in the context of cancer?
It refers to the methylation of tumor suppressor genes, preventing their transcription and contributing to cancer progression.
What is the function of the TP53 protein?
It regulates the cell cycle, DNA repair, and apoptosis, acting as a tumor suppressor.
What is the significance of the RB1 gene?
It inhibits the cell cycle; mutations can lead to retinoblastoma, either bilateral (inherited) or unilateral (somatic mutations).
What are BRCA1 and BRCA2 associated with?
Hereditary Breast and Ovarian Cancer (HBOC), where mutations increase the risk of breast and ovarian cancers.
What is the duration of pregnancy from fertilization?
266 days, or 280 days from the last menstrual cycle.
What occurs during the embryonic period of pregnancy?
Weeks 1-8, during which the embryo is most susceptible to teratogens.
What is the acrosome reaction?
The release of enzymes from the sperm that help it penetrate the zona pellucida of the egg.
What is the significance of the cortical granule reaction?
It prevents polyspermy by modifying the zona pellucida to make it resistant to additional sperm.
What is implantation in embryology?
The process where the blastocyst attaches to the endometrium of the uterus, occurring around day 6 after fertilization.
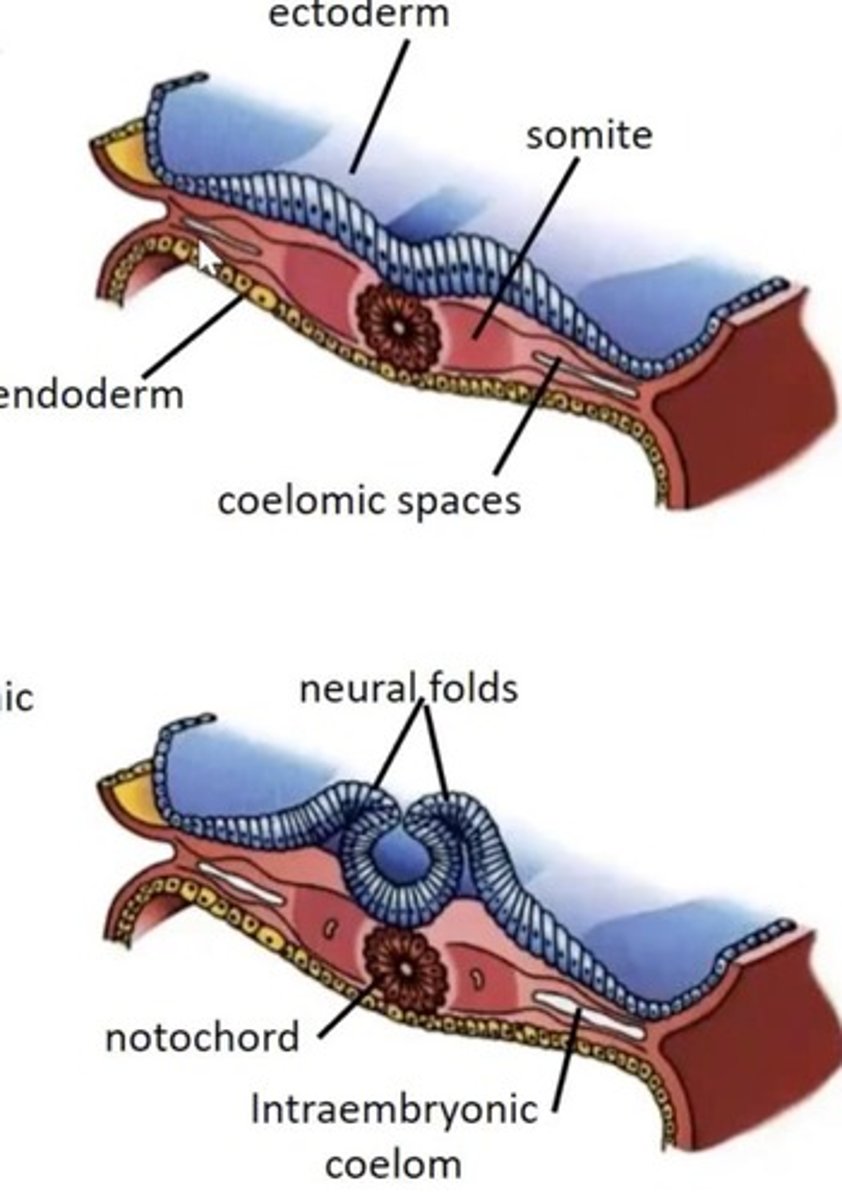
What is the role of the Notochord?
It is formed from cells migrating through the primitive pit and plays a critical role in the development of the vertebrate body plan.
What is the function of the notochord?
Induction of the nervous system and stimulation of vertebral body formation.
What condition results from failure of vertebral column formation?
Spina bifida.
What do the neural folds develop into?
The neural tube.
What do neural crest cells give rise to?
The peripheral nervous system, melanocytes, endocrine secretory cells, and jaw.
What are the derivatives of the endoderm?
Lining of the gastrointestinal and respiratory tracts, and epithelial components of accessory organs like the pancreas and liver.
What are the three cavities formed from the lateral plate mesoderm?
Pericardial, pleural, and peritoneal cavities.
What initiates embryonic folding during gastrulation?
The growth of the neural tube.
What is the role of maternal effect genes in embryonic development?
They activate zygotic genes and establish gradients for anterior-posterior axis formation.
What is the function of the Bicoid gene?
It drives the formation of anterior structures in the embryo.
What happens when Bicoid mRNA is absent?
The embryo develops without head or thorax structures, resulting in two tails.
What is the role of the Dorsal gene in Drosophila development?
It codes for a morphogen that activates the dorsal transcription factor, critical for dorsal-ventral axis specification.
What is the significance of the gradient formed by Spätzle and Toll proteins?
It determines the activation of the Dorsal transcription factor in the embryo.
What are the five classes of genes involved in establishing the body plan?
Maternal effect genes, gap genes, pair-rule genes, segmentation polarity genes, and homeotic selector genes.
What is the function of gap genes?
They divide the embryo into broad regions and initiate the development of specific areas.
What is the effect of high levels of Hunchback on Kruppel expression?
High levels of Hunchback lead to very low levels of Kruppel, inhibiting its transcription.
What is the role of Nanos mRNA in embryo development?
It blocks the translation of Hunchback maternal mRNA, regulating anterior-posterior axis formation.
What is the result of dorsal-ventral axis patterning failure?
It leads to a non-viable embryo due to improper division into dorsal and ventral regions.
What is the purpose of rescue experiments in developmental biology?
To restore anterior-posterior patterning by transferring mRNA from wild-type to mutant embryos.
What is the outcome of the sequential expression of maternal effect genes?
They activate zygotic genes that establish the body plan of the embryo.
What happens during lateral folding of the embryo?
It incorporates the yolk sac into the embryo, forming the gut and surrounding the embryo with the amnion.
What do somites differentiate into?
Sclerotome (vertebrae and ribs) and dermatomyotome (skeletal muscles and connective tissue).
What is the significance of the cephalic furrow?
It separates the head from the abdomen and is controlled by the amount of Bicoid mRNA.
What is the role of the intermediate mesoderm?
It gives rise to the genital tract and urinary system.
What is the function of the lateral plate mesoderm?
It develops into smooth muscle, most connective tissue, and the cardiovascular system.
What are pair rule genes and name three key examples?
Pair rule genes are activated after every second parasegment, with key examples being hairy, even skipped, and runt.
What is the role of enhancer genes in stripe formation?
Enhancer genes determine the specific stripe number that the even-skipped gene codes for.
How do bicoid and hunchback affect even-skipped gene transcription?
Bicoid and hunchback binding to DNA stimulate the transcription of the even-skipped gene.
What is the function of segment polarity genes?
Segment polarity genes regulate the transcription of specific genes after cellularization, defining the boundary and polarity of segments.
What are the consequences of mutations in segment polarity genes?
Mutations can interfere with adult development and lead to non-viable embryos.
What is the definition of segment identity in embryonic development?
Segment identity refers to what each segment of the embryo turns into, determined by Hox genes.
What are homeotic selector genes responsible for?
Homeotic selector genes encode transcription factors that control segment identity.
What happens when Hox genes are deleted?
Deletion of Hox genes can cause a segment to differentiate into an adjacent segment.
What is homeosis in developmental biology?
Homeosis is the phenomenon where the development of one body part has the phenotype of another body part.
What is the significance of the HomC-complex in insects?
The HomC-complex is an arrangement of homeotic selector genes found in insects.
What are the properties of stem cells?
Stem cells have the ability to proliferate, differentiate, and self-renew.
What are the three types of stem cells?
Unipotent (can only reproduce themselves), totipotent (can become any type of cell), and pluripotent (can become multiple cell types).
What defines the differentiation process of stem cells?
Differentiation is unidirectional, moving from stem cells to germ layers and then to all major cell types.
How are developmental strategies conserved across species?
Developmental strategies are ancient and highly conserved, with homologs of selector genes found in all animals.
What is the role of engrailed (en) in segment polarity?
Engrailed is a transcription factor that binds to the promoter of the hedgehog gene, activating the hedgehog pathway.
What is the relationship between wingless (wg) and engrailed (en)?
Wingless activates engrailed genes in adjacent cells, contributing to boundary signaling.
What is the significance of the homeobox domain in homeotic genes?
The homeobox domain is responsible for directing where transcription factors bind to DNA.
What happens when anterior Hox genes are mutated?
If an anterior Hox gene is mutated, the anterior gene to the mutated gene is expressed instead.
What is the effect of misexpression of antennapedia (antp) in anterior segments?
Misexpression of antp can lead to legs forming where antennae should be.
What are the three types of stem cell potency?
Pluripotent (all somatic cell types except extraembryonic tissues), Multipotent (specific somatic cell types), Bipotent (only 2 cell types).
What is the significance of asymmetric cell division in stem cells?
It results in the unequal distribution of cell contents, leading to terminal differentiation.
What is quiescence in the context of stem cells?
A state where cells are not replicating, often referred to as the G0 phase.
What triggers stem cell activation and proliferation?
Tissue damage serves as a trigger for stem cell activation.
What is terminal differentiation?
A process where a stem cell cannot return to S-phase and must be replaced by other cells.
What are the consequences of forced division in stem cells?
It can lead to necrosis or mitotic catastrophe, which serves as a cancer protection mechanism.
What is the role of the stem cell niche?
It provides a protective environment and signals that regulate stem cell function.
What are the four main signals that regulate stem cell behavior?
Growth factors, cytokines, transcription factors, and ligand-receptor interactions.
What is the function of Notch signaling in stem cells?
It inhibits differentiation and maintains the quiescent state of stem cells.
What are the characteristics of adult stem cells?
They are multipotent, found in every tissue type, and require a niche for protection.