Chapter 11: Catabolism Energy Release and Coservation
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
87 Terms
Requirements for C, H, O
- often satisfied together (in the same molecule)
- Some organisms have great metabolic flexibility which depends on environment
Energy source
- phototrophs use light
- chemotrophs obtain energy from oxidation of chemical compounds
chemotrops
obtain energy from chemicals
Electron source
- organotrophs use organic compounds
- Lithotrophs used reduced inorganic substances
organotrophs
obtain electrons from organic compounds
Lithotrophs
use inorganic molecules as a source of electrons
Carbon Source
- heterotrophs use organic molecules, which often also serves as energy source
- autotroph use a single carbon molecule
heterotroph
use organic molecules which serve as carbon, electron and energy source all in one
autotroph
use a single carbon molecule (like CO2)
Photolithoautotroph
Carbon Source: CO2
Energy Source: Light
Electron Source: Inorganic e- donor
Photoorganoheterotroph
carbon source: organic carbon
energy source: light
electron source: organic e- donor
Chemolithoautotrophs
Carbon Source: CO2
Energy Source: Inorganic chemicals
Electron Source: Inorganic e- donor
Chemolithoheterotroph
Carbon Source: organic carbon
Energy Source: Inorganic chemicals
Electron Source: Inorganic e- donor
Chemoorganoheterotroph
Carbon Source: organic carbon
Energy Source: organic carbon
Electron Source: organic chemicals (often same as C source)
other names for chemoorganoheterotrophs
chemoheterotrophs and chemoorganotrophs
Which bacteria is both a phototroph and chemotroph
cyanobacteria
The majority of microorganisms known are what type of nutritional type?
- photolithoautotrophs/photoautotroph (inorganic)
- chemolithoautotrophs (inorganic)
- chemoorganoheterotrophs/ chemoheterotrophs/ chemoorganotrophs (organic)
Where are the majority of pathogens found?
chemoorganoheterotroph where single organic nutrient can satisfy all three requirements
What are the basic needs of all organisms?
- ATP as energy source
- reducing power to supply electrons for chemical reactions
- precursor metabolites for biosynthesis (Carbon -> monomers)
Inorganic electron donors
H2, H2S, Fe2+, NH4+
Chemoorganotrophs donate their electrons for what?
Fermentation
Aerobic respiration
Anaerobic respiration
Chemolithotrophs donate their electrons for what?
Aerobic respiration
Anaerobic respiration
Chemoorganotrophic Fueling Process
- Organic energy source is oxidized, it's electrons are passed to NADH/FADH2
- Respiration if electrons are donated to ETC
- Fermentation if electrons are donated to endogenous acceptor (like pyruvate)
- Catabolism because it releases energy and provides carbon and electrons for anabolism
What is the difference between fermentation and respiration?
- It has NOTHING to do with oxygen
- Presence of Electron Transport Chain (ETC) and Electromotive Force (emf)
> Respiration: Uses an ETC to transfer electrons from NADH/FADH₂ to a final electron acceptor (which can be oxygen in aerobic respiration or another molecule in anaerobic respiration). The movement of electrons creates an electromotive force (emf) that drives oxidative phosphorylation, generating ATP.
> Fermentation: Lacks an ETC. Instead, it relies only on substrate-level phosphorylation (glycolysis) for ATP production.
- Fates of NADH and Pyruvate
> Respiration: NADH donates electrons to the ETC. Pyruvate is completely oxidized into CO₂ (in aerobic respiration, via the citric acid cycle).
> Fermentation: Since there is no ETC. NADH must be recycled directly by transferring electrons to pyruvate or its derivatives. Pyruvate is not fully oxidized; instead, it is converted into fermentation products like lactate (in lactic acid fermentation) or ethanol and CO₂ (in alcoholic fermentation).
Aerobic respiration
completely catabolize an organic energy source to CO2 via:
1) Glycolysis to produce 2 pyruvates
- Net gain: 2 ATP, 2NADH per glucose
2) TCA Cycle to completely oxidize pyruvate to CO2
- Net gain: 4 NADH, 1 ATP, 1 FADH2 per pyruvate
3) ETC with oxygen as the final electron acceptor
What are the 3 common routes of the central metabolic pathways?
1) Embden-Meyerhof pathway (EMP, Glycolysis)
2) Entner-Duodoroff pathway (ED)
- special to some bacteria
3) Pentose phosphate pathway (PPP)
- Works at the same time as EMP or ED
- makes the 5C skeleton for biosynthesis of nucleotides
What do the three central pathways EMP, ED, and PPP have in common?
- convert glucose to glyceraldehyde-3-phosphate (GAP)
- GAP is oxidized to pyruvate the same way
Net gain of EMP pathway
2 ATP, 2 NADH per glucose
net gain of ED pathway
1 (?) ATP, 1 NADH, 1 NADPH per Glucose
net gain of PPP pathway
1 (?) ATP, 2 NADH
Which of the 12 carbon skeletons are produced in the central pathways?
FIVE Carbon Skeletons from EMP/ED/PPP
- Glucose-6-phosphate (6C)
- Glyceraldehyde-3-phosphate or GAP (3C)
- 3-phosphoglycerate or 3PG (3C)
- Phosphoenolpyruvate or PEP (3C)
- Pyruvate
TWO Carbon Skeletons from PPP only
- Ribose-5-phosphate (5C)
- Erythrose-4-Phosphate (5C)
What enzyme does the following reaction:
Gluc --> G6P
Hexokinase
3 multiple choice options
What enzyme does the following reaction:
F6P --> FBP
Phosphofructokinase
3 multiple choice options
What enzyme does the following reaction:
PEP --> Pyruvate
Pyruvate Kinase
3 multiple choice options
What enzyme does the following reaction:
G6P --> Glucose
Glucose-6-phosphatase
3 multiple choice options
What enzyme does the following reaction:
FBP --> F6P
Fructose 1,6-biphosphatase
3 multiple choice options
What enzyme does the following reaction:
Pyruvate --> PEP
PEP Carboxykinase
Pyruvate carboxylase
3 multiple choice options
Which pathways are amphibolic?
- EMP/glycolysis
- TCA cycle
- PPP
What are amphibolic pathways?
functions both as catabolic and anabolic pathways
central amphibolic pathways can be anabolic or catabolic based on what?
ATP, PEP, and F6P levels
What is the goal of glycolysis?
To turn Glucose into Pyruvate, so it can enter into the Krebs cycle to produce more energy and generate ATP (Energy) in the process.
What is the goal of the TCA cycle?
- fully oxidize the energy source (glucose) into CO2
- Generate reducing power NADH, FADH2
What is the pyruvate dehydrogenase complex (PDHC)?
- oxidizes and decarboxylates pyruvate into acetyl-CoA and CO2
- This process generates and NADH
Which of the 12 carbon skeletons are produced in the TCA cycle?
FOUR of the carbon skeletons
- Acetyl-CoA (2)
- a-ketoglutarate (5C)
- Succinyl-CoA (4C)
- Oxaloacetate (4C)
net gain of TCA cycle
3 NADH (+ 1 NADH from PDHC), 1 FADH2, 1 ATP/GTP per pyruvate
Why is acetyl-CoA important?
fatty acids are synthesized from this molecule outside the mitochondrion, in the cytosol. These can then become membrane lipids.
Why is a-ketoglutarate important?
it is the intermediate to adding amino group to the carbon skeletons
Why is oxaloacetate important?
it is the precursor to 6 amino acids and nitrogenous bases
How many rounds must the TCA cycle go through for one glucose molecule?
2 times
3 multiple choice options
ETC makes most of ATP as _______
NADH and FADH2 are re-oxidized
ETC is a series of electron carriers that move from ______ to _______
More negative reduction potential; more positive
ATP is made from ____ powered by pmf
Oxidative phosphorylation
Rotenone
Blocks NADH Dehydrogenase (complex I) and NADH accumulates.
Malonate or 3-nitropropionate
competitive inhibitor of succinate dehydrogenase (Complex II)
beta oxidation
A metabolic sequence that breaks fatty acids down to two-carbon fragments that enter the citric acid cycle as acetyl CoA.
How is prokaryotic ETC different from eukaryotic ETC?
- Located on the cell membrane
- Different electron carriers
- may be branched
- may be shorter
- lower P/O ratio
e.coli
facultative anaerobic bacterium, gram-negative, rod, can ferment
What are the ETC pathways of e.coli when oxygen is present?
1) bd branch - low oxygen available
2) bo branch - high oxygen available
BD Branch of E. coli ETC
stationary phase and low aeration, higher affinity for oxygen, moves fewer protons
NADH e' donor -> NADH dehydrogenase -> Quinone --> cytochrome bd oxidase --> O2
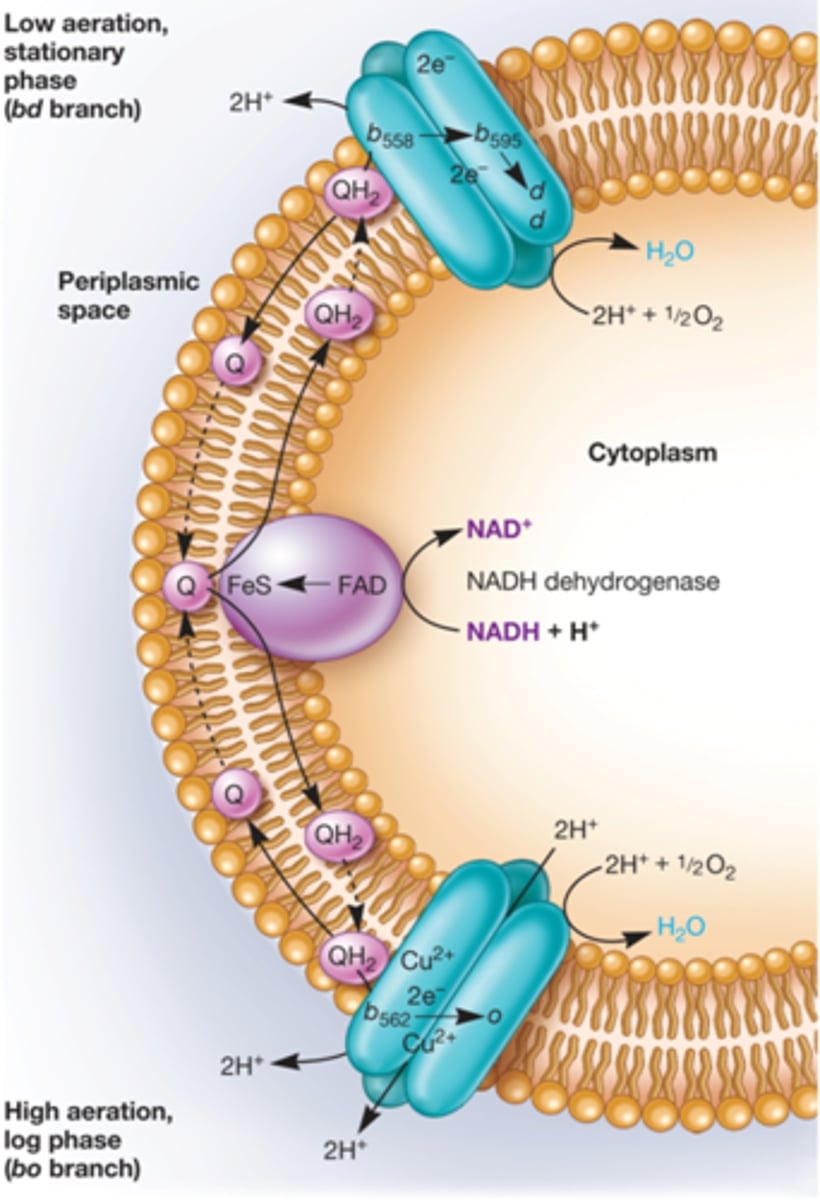
BO branch of E. coli ETC.
log phase and high aeration, lower affinity for oxygen (more H+ pumped)
NADH e' donor -> NADH dehydrogenase -> Quinone --> cytochrome bo oxidase --> O2
Anaerobic respiration of e.coli
1) Fumarate reductase (Fumarate -> succinate)
2) Nitrate reductase (NO3- --> NO2- --> NH4+)
Paracoccus denitrificans
- gram-negative facultative anaerobe, soil bacterium, non fermenting
-extremely versatile metabolically
- under aerobic conditions, uses aerobic respiration
- can use one-carbon molecules instead of glucose as a source of energy and electrons
Paracoccus denitrificans in aerobic respiration
- similar electron carriers and transport mechanism as mitochondria
- protons transported to periplasmic space rather than inner mitochondrial membrane
Paracoccus denitrificans ETC when using 1-carbon molecule (methanol)
no NADH involved, e- donated to cyt c via methanol dehydrogenase (MD)
In ATP synthase, F0 is
proton channel
In ATP synthase, F1 is
uses the energy released by the gradient to phosphorylate ADP into ATP
Theoretical maximum total yield of ATP during aerobic respiration
32 ATP (30 in eukaryotes)
Most common electron acceptors for anaerobic respiration
nitrate, sulfate and carbon dioxide
Paracoccus denitrificans anaerobic respiration
- dissimilatory nitrate reduction/denitrification
- NO3-, NO2-, NO, N2O, N2
- enzymes inhibited by O2
- Nitrate is the terminal electron acceptor
- Also done by Pseudomonas and Bacillus (facultative anaerobes)
Fermentation
- energy source is partially oxidized
- Oxidation of NADH from glycolyses
--> NADH is converted back to NAD+
--> pyruvate or derivative e- acceptor
- Oxygen NOT needed
- No ETC or OP
--> ATP formed by SLP only
Fermentation does not cause
pmf through ETC but in other ways
Work without ETC (generating pmf)
1) Fermentation: end-product efflux
2)Facultative anaerobes: pmf redox-loop mechanism
3) Strict fermentative conditions: F1Fo-ATP synthase operates reversibly
pmf redox-loop mechanism
it takes 2 enzymes to pump H+
Common Microbial Fermentation
1) Mixed acid fermenters (e.coli)
2) Butanediol fermenters (Enterobacter)
3) Alcoholic acid fermenters
What media can you use to differentiate between mixed acid fermenters and butanediol fermenters?
EMB
green = mixed
Lavender = butanediol
hydrolases
catalyze cleavage with the addition of water
found outside
catabolism of disaccharides and polysaccharides
Phosphorylases
introduces a phosphate group into an organic molecule
disaccharide and polysaccharide catabolism
found inside
triglycerides can be used by
chemoorganotrophs
Triglycerides are hydrolyzed by
lipases
Lipases in lipid catabolism
1) glycerol degraded via glycolytic pathway as dihydroxyacetone phosphate --> GAP
2) Fatty acids oxidized via b-oxidation pathway which shortens it 2 carbons at a time (acetyl-CoA)
proteases
enzymes that break down proteins to amino acids
Deamination followed by transamination
result in organic acids converted to pyruvate, acetyl-CoA, or TCA cycle intermediate
e- donor in chemolithotrophy
from inorganic molecule: H2, reduced nitrogen, reduced sulfur, and Fe2+
donated directly to the ETC
Chemolithotrophy terminal e- acceptor
oxygen, sulfate and nitrate
Do chemolithotrophs use fermentation?
no
3 major groups of chemolithotrophs
1) Several bacteria and archaea oxidize hydrogen: reduce NAD+ or donate directly to ETC
2) Nitrifying bacteria found in soil and water carry out nitrification: oxidation of ammonia (NH3) to nitrate (NO3) through a two step process from two different microbes or one
3) Sulfur-oxidizing microbes: oxidize hydrogen sulfide (H2S), Sulfur, thiosulfate (S2O3) and other reduced sulfur compounds to sulfuric acid (H2DO4)
autotrophs reverse electron flow
- need NAD(P)H and ATP to reduce CO2
- cannot donate electrons directly to NAD(P)+