Mass Spectrometry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What is Mass Spectrometry?
An analytical technique used to identify chemical substances by sorting gaseous ions based on their mass-to-charge ratios.
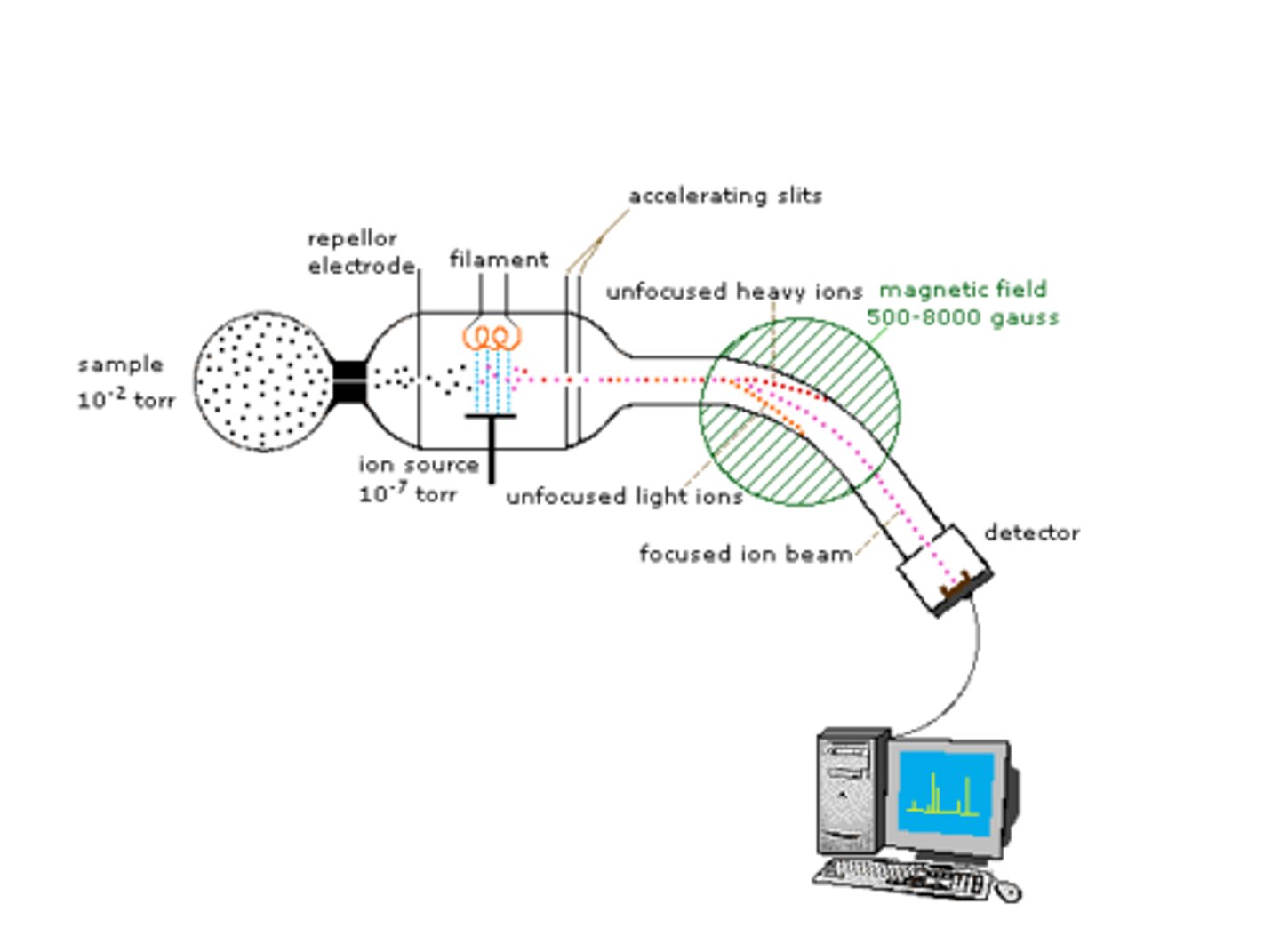
How Does Mass Spectrometry Work?
The molecules in the small sample are bombarded with high energy electrons which can cause the molecule to lose an electron.
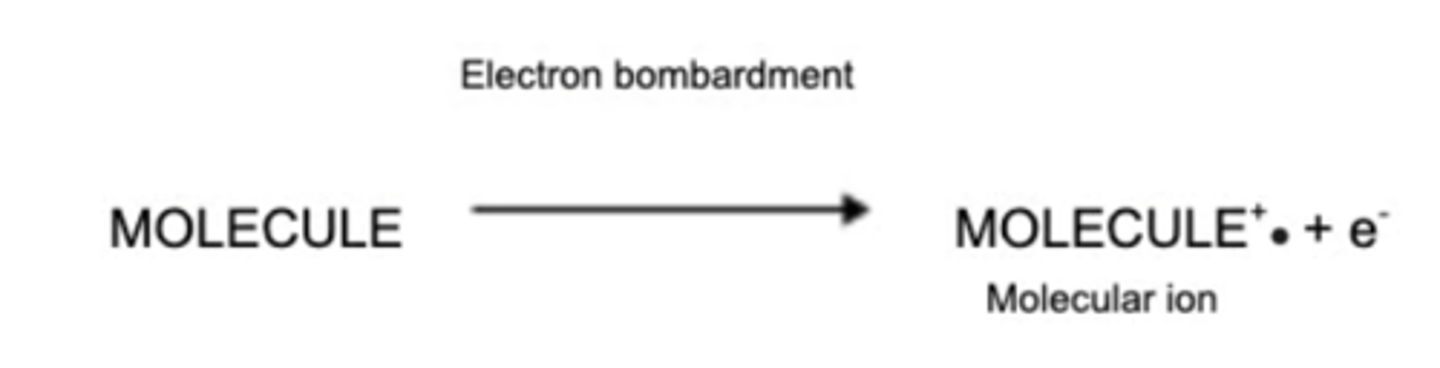
How is the Molecular Ion Determined?
In the mass spectrum, the heaviest ion (the one with the greatest m/z value) is likely to be the molecular ion.
What is the Molecular Ion?
The highest-mass ion in a spectrum is normally considered to be the molecular ion. This can fragment to form new ions, molecules, and radicals
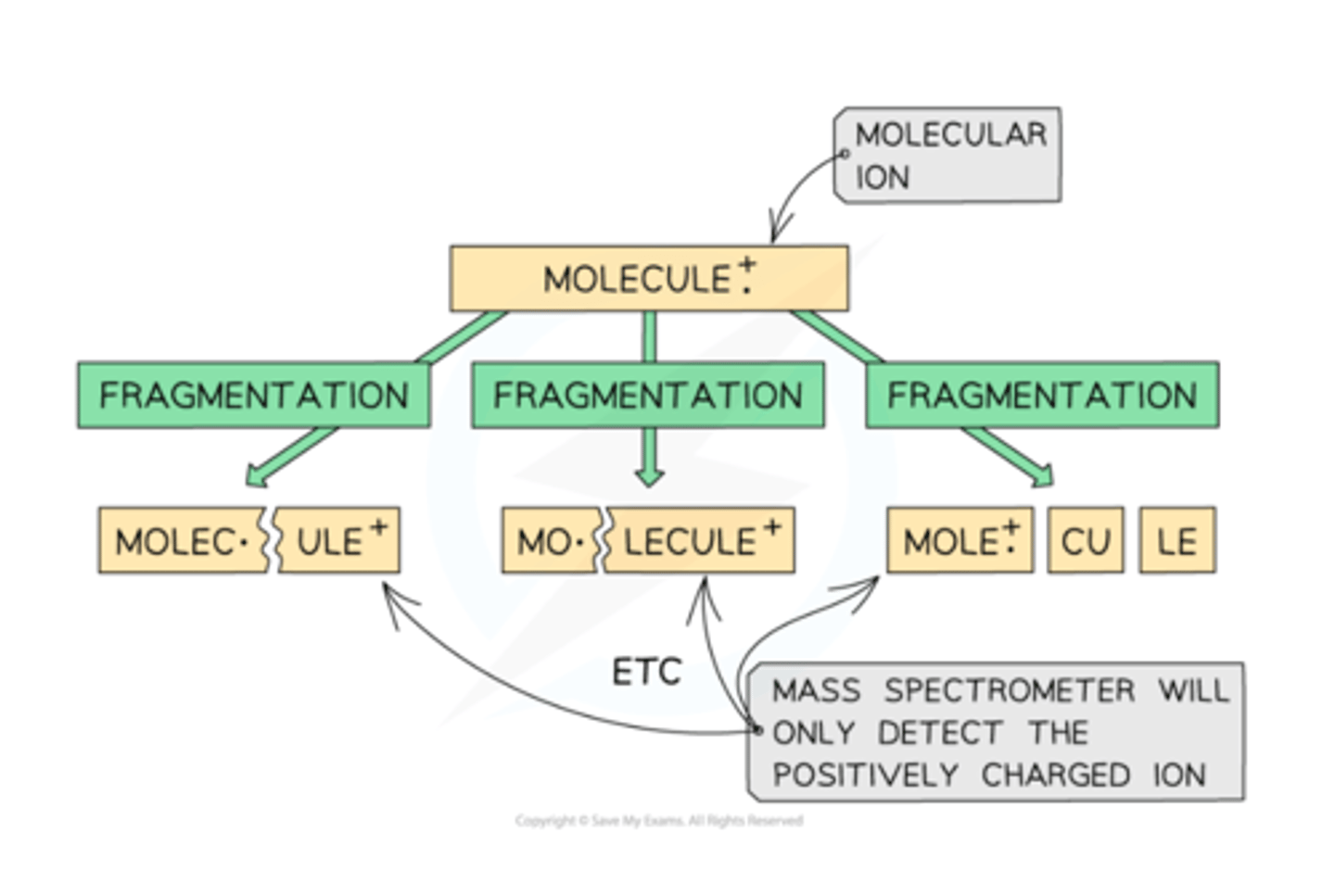
How are Molecular Ion Fragments Separated?
Based on their mass (m) to charge (z) ratio, the ion fragments are then separated by deflecting them into the detector.
Most ions will only gain a charge of 1+ and therefore a ion with mass 12 and charge 1+ will have an m/z value of 12.
It is, however, possible for a greater charge to occur. For example, an ion with mass 16 and charge 2+ will have a m/z value of 8.
How do you Determine the Relative Molecular Mass of an Organic Compound?
Look for the peak with the highest value for m/z.
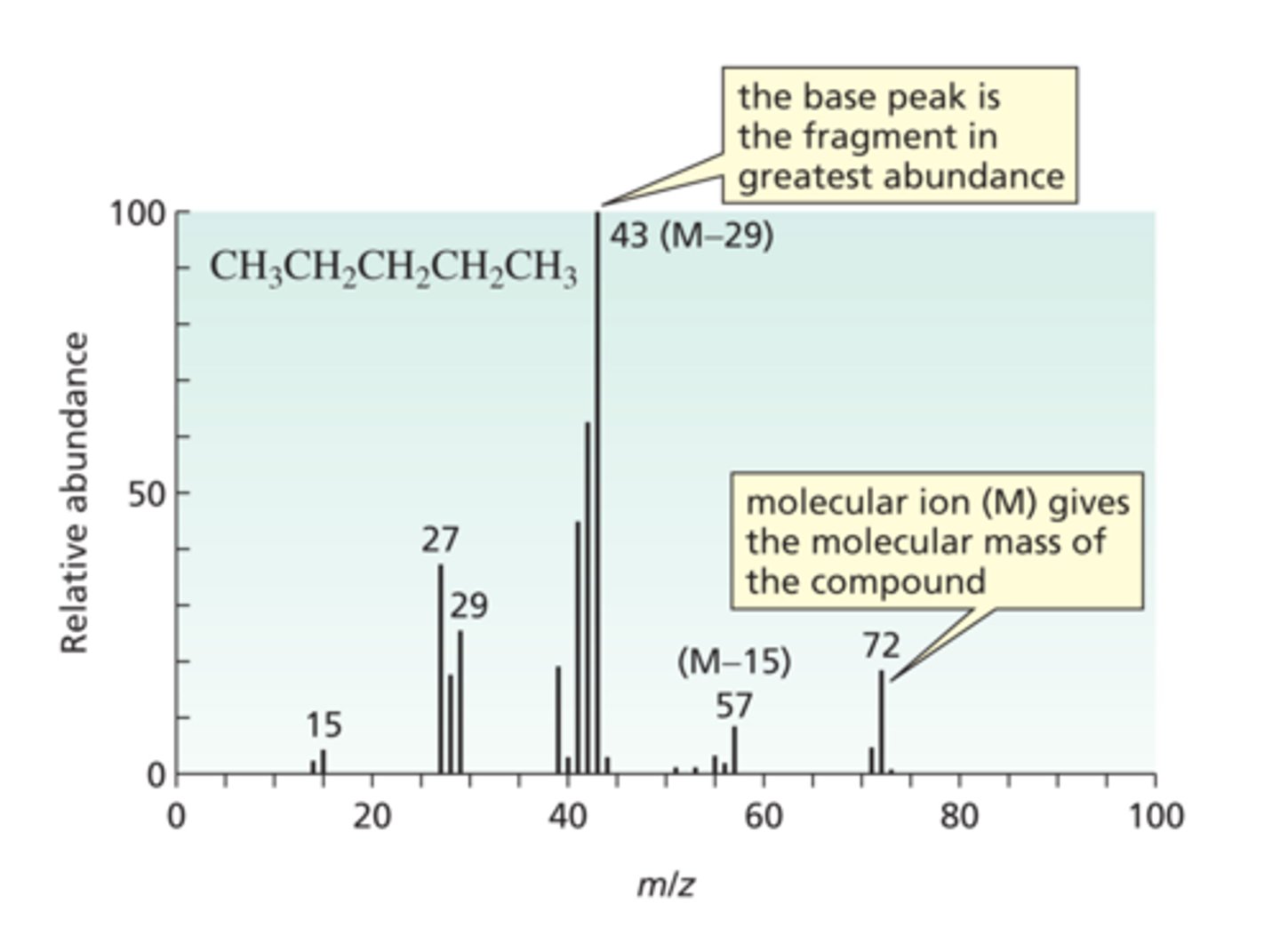
What is the m/z sometimes called?
m/e ratio and it is almost always 1:1.
In a sample of iron, the ions 54Fe2+ and 56Fe3+ are detected. Calculate their m/z ratio and determine which ion is deflected more inside the spectrometer.
56Fe3+ has a smaller m/z ratio and will therefore be deflected more.
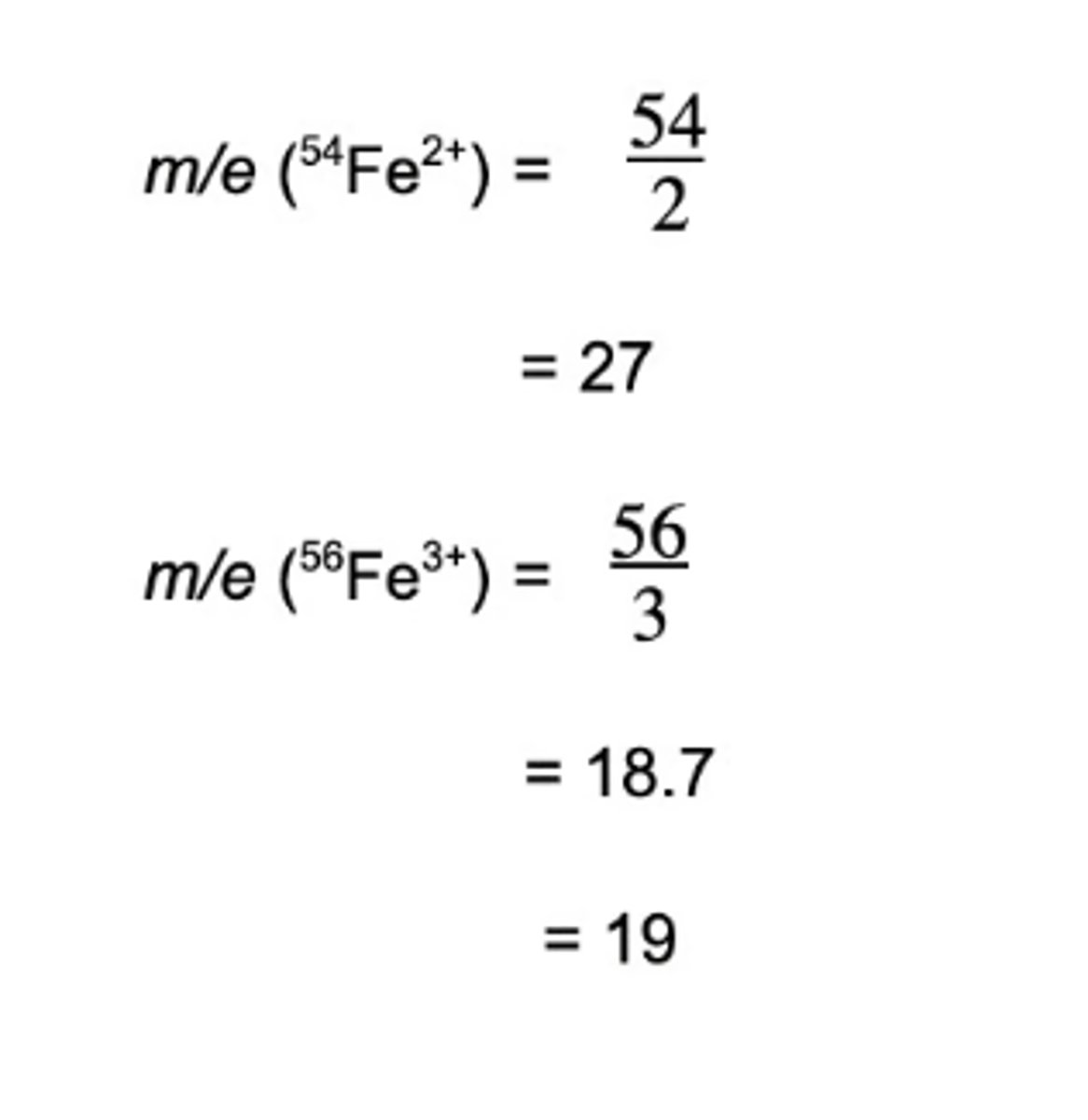
What is the M+1 Peak?
Smaller peak which is due to the natural abundance of the isotope carbon-13.
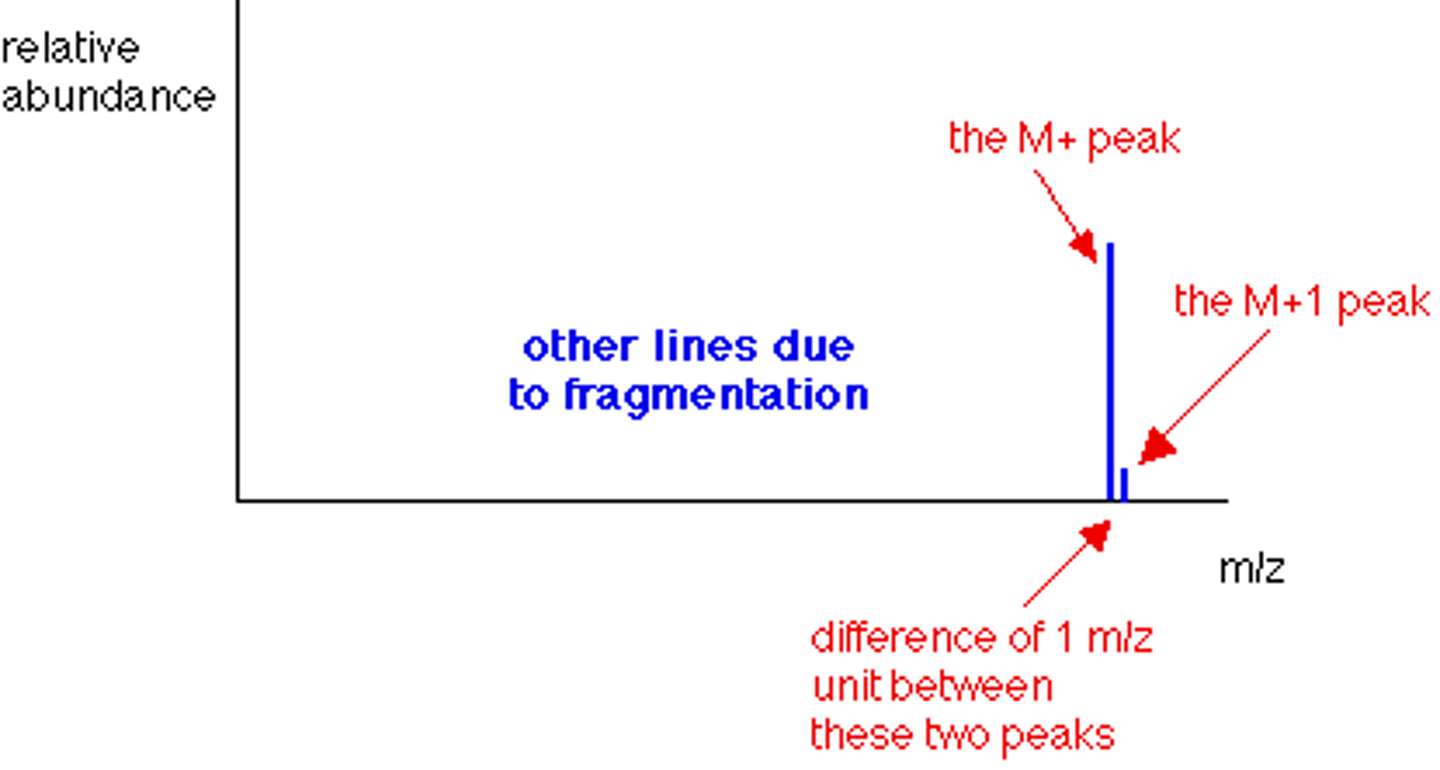
What does the Height of the M+1 Peak Depend on?
How many carbon atoms are present in that molecule; the more carbon atoms, the larger the [M+1] peak is.
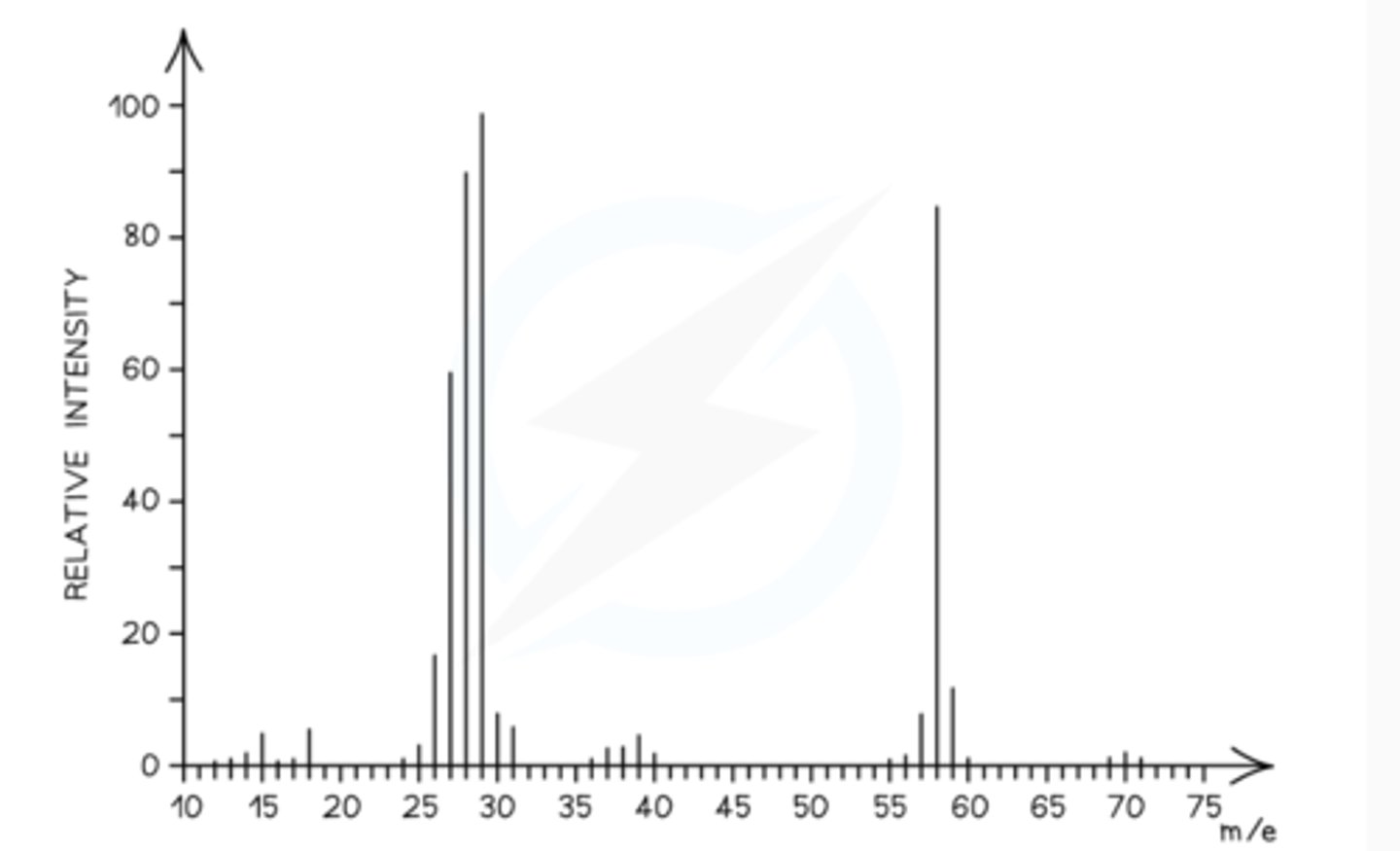
Determine whether the following mass spectrum corresponds to but-1-ene or pent-1-ene:
The mass spectrum corresponds to pent-1-ene as the molecular ion peak is at m/z = 70
The small peak at m/z = 71 is a C-13 peak, which does not count as the molecular ion peak
But-1-ene arises from the C4H8+ ion which has a molecular mass of 56
Pent-1-ene arises from the C5H10+ ion which has a molecular mass of 70
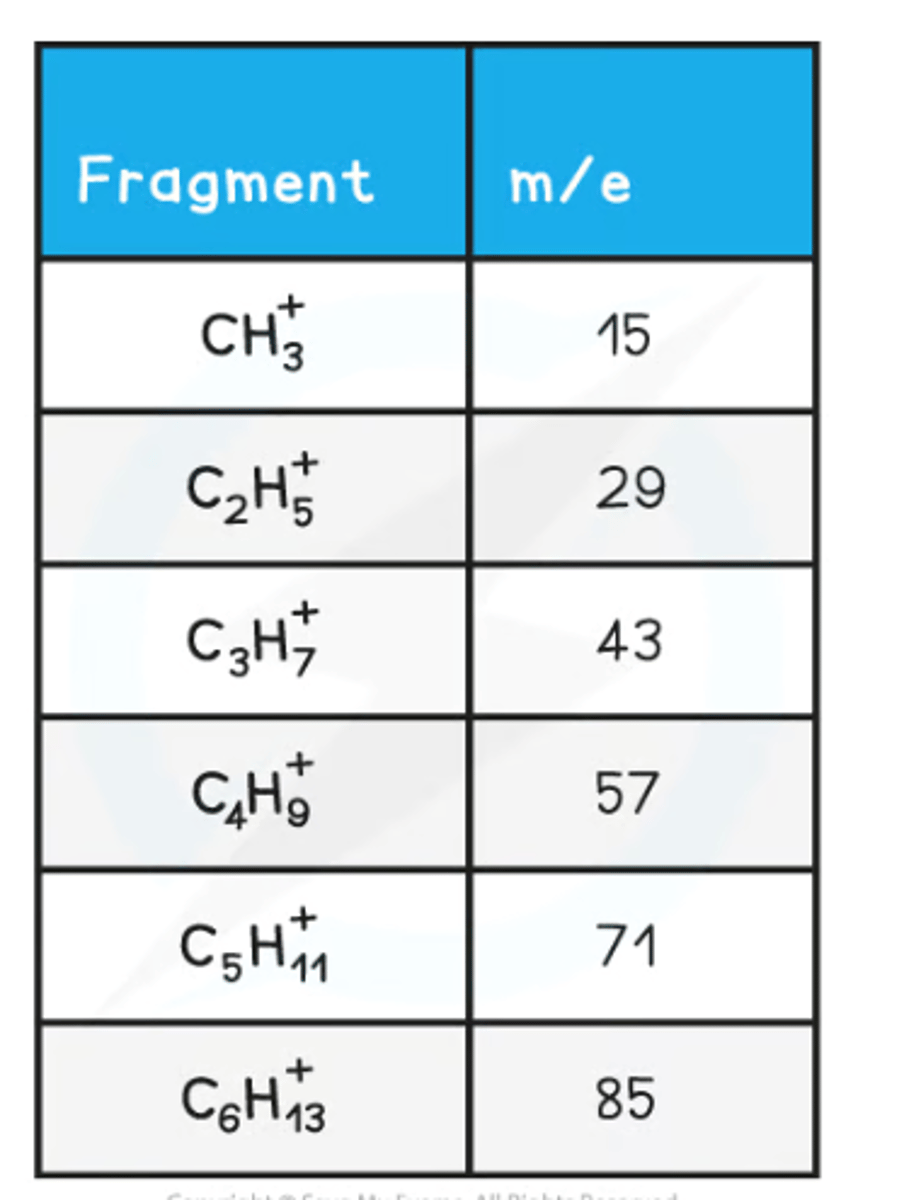
How are Simple Alkanes Fragmented?
Simple alkanes are fragmented in mass spectroscopy by breaking the C-C bonds.
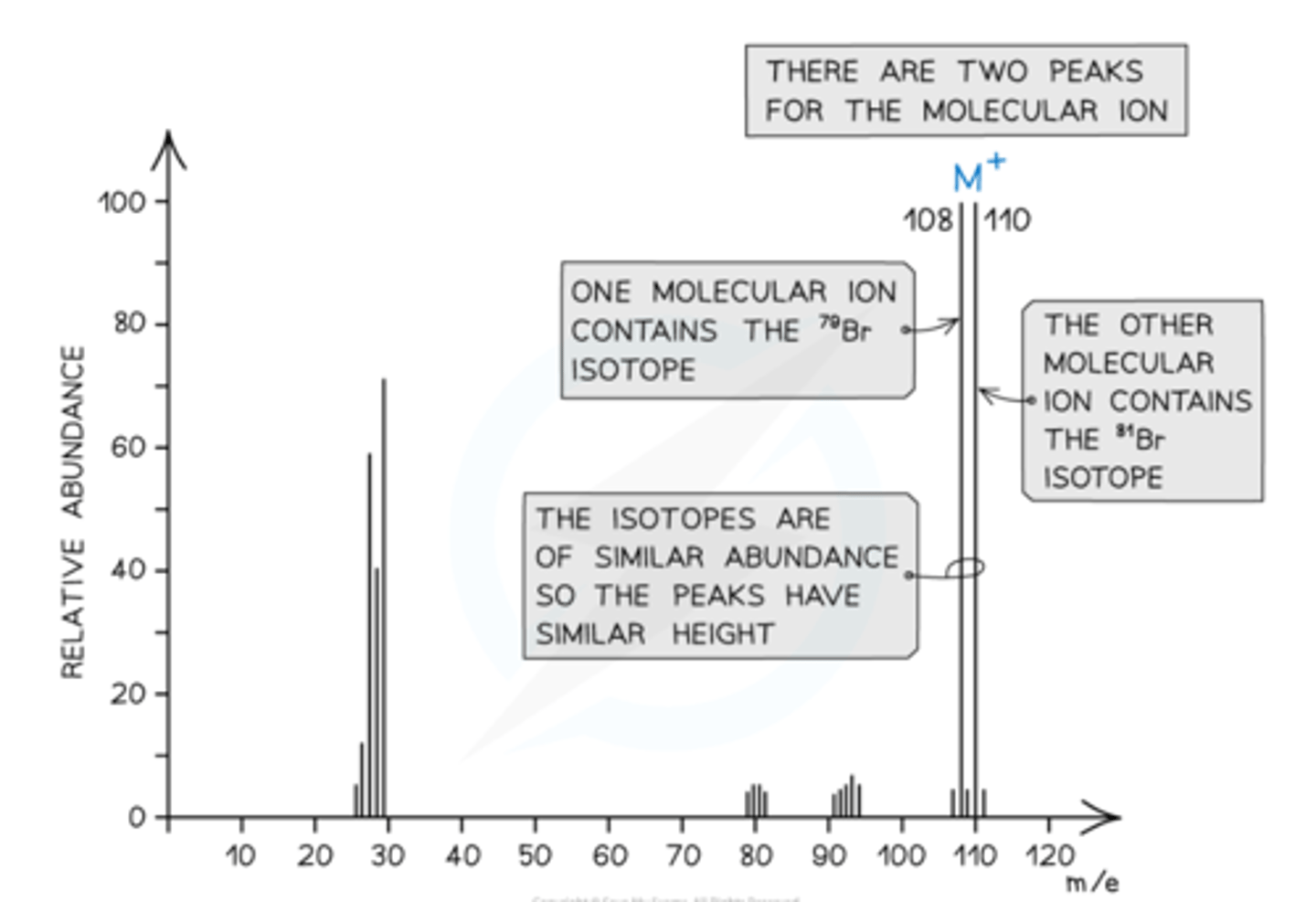
How are Fragmentation Patterns shown in halogenoalkanes?
Halogenoalkanes often have multiple peaks around the molecular ion peak.
This is caused by the fact that there are different isotopes of the halogens.
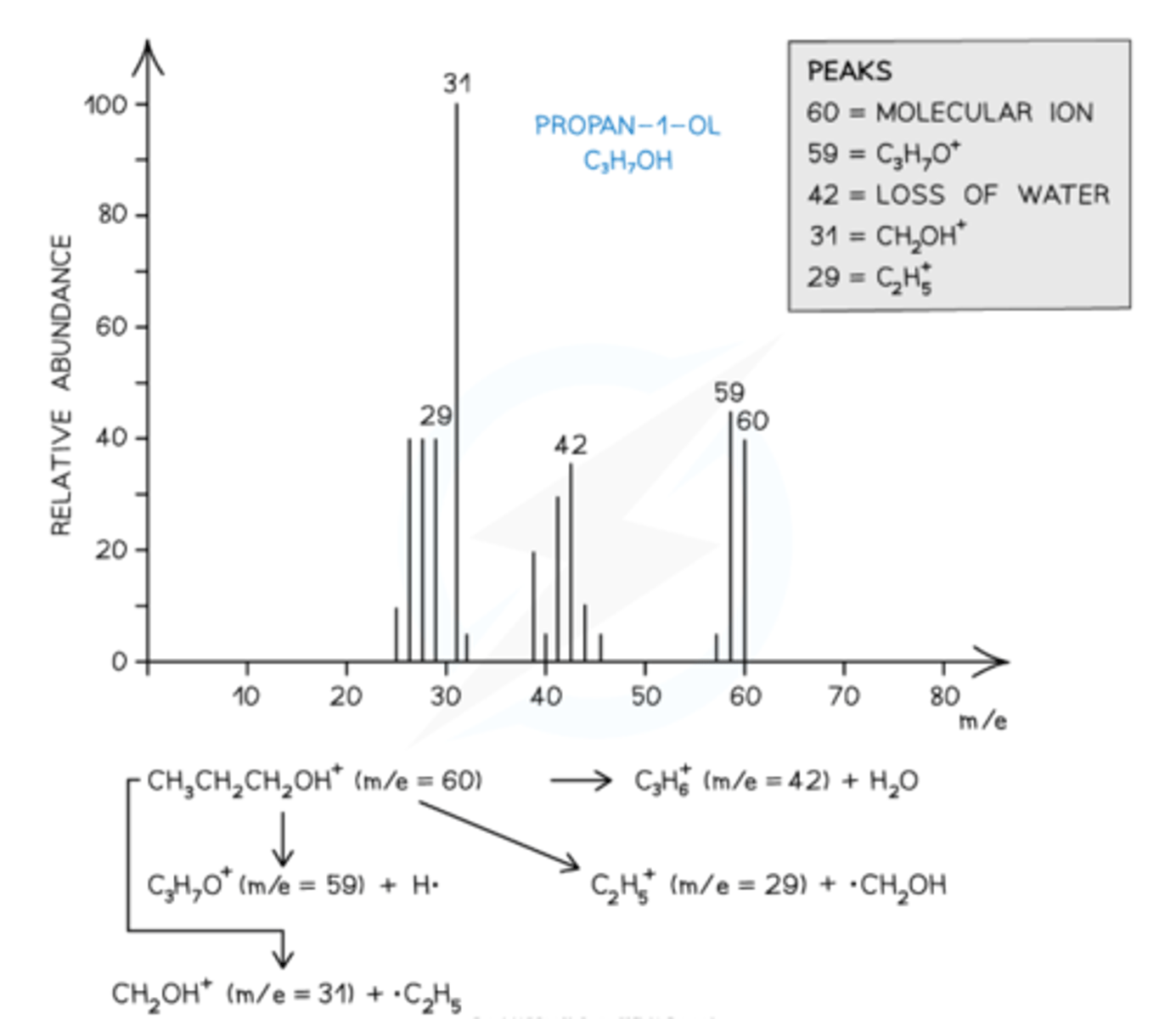
How are Fragmentation Patterns shown in Alcohols?
- lose a H2O molecule giving rise to a peak at 18 below molecular ion.
- common peak found at m/e value 31 (CH2OH+ fragment).
Mass spectrum showing the fragmentation patterns in propan-1-ol (alcohol)
Which of the following statements about the mass spectrum of CH3Br is correct?
A. There is one peak for the molecular ion with an m/e value of 44
B. There is one peak for the molecular ion with an m/e value of 95
C. The last two peaks have abundances in the ratio 3:1 and occur at m/e values of 94 and 96
D. The last two peaks are of equal size and occur at m/e values of 94 and 96
D.
Bromomethane (CH3Br) can produce 3 peaks
CH381Br → [CH381Br]+ + e− at m/e 96
CH379Br → [CH379Br]+ + e− at m/e 94
CH3Br → [CH3]+ + •Br at m/e 15
![<p>D. </p><p>Bromomethane (CH3Br) can produce 3 peaks</p><p>CH381Br → [CH381Br]+ + e− at m/e 96</p><p>CH379Br → [CH379Br]+ + e− at m/e 94</p><p>CH3Br → [CH3]+ + •Br at m/e 15</p>](https://knowt-user-attachments.s3.amazonaws.com/7c5fc95e-3be2-471c-ae9d-cccdcfb980bd.jpg)