Chapter 21 - Nutrient cycles
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
What determines the purpose for which microbes use certain elements
The chemical state
Environmental conditions
Major carbon reservoirs on earth
Rocks and sediments
Oceans
methane hydrates
Fossil fuels
Atmosphere composition
N2 = 78%
O2 = 21%
CO2 = 0.039%
Other gasses
How much ppm of CO2, is in the air.
400 ppm.
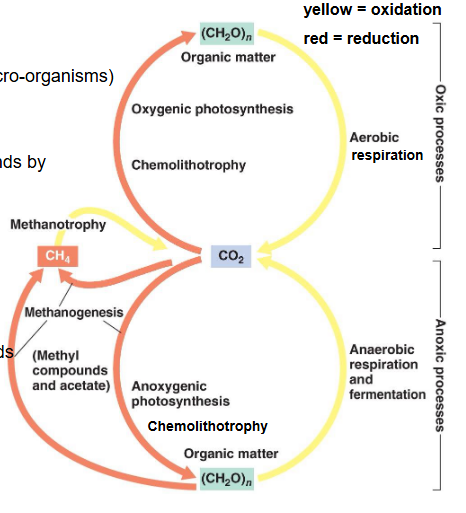
Carbon cycle
CO2 goes in the air from fossil fuels, animals and microbes.
Is taken up by plants
Is taken up by the water for aquatic plants.
Relation between organic matter and CO2 in carbon cycle
KNOW THIS
Methanogens vs. Methanotrophs
Methanogens produce CH4 by reducing CO2
Methanotrophs use CH4 as an electron donor and oxidize it to form CO2
Methanogenesis
All are archaea
Strictly anaerobic
Are diverse in morphology and circumstances under which they occur.
Can use H2 (donor) as an energy source and then breathe with CO2 (acceptor) creating CH4
The three methanogenic pathways and their substrates
CO2 reduction pathway, electrons are typically derived from H2
Acetolactic pathway: Acetate or pyruvate is used
Very few: electron donor is methylated single carbon molecules.
Methane vs. CO2
Methane contributes twice as much to pollution compared to CO2
Largest methane sources
Ruminants
Termites
Paddy fields (freshwater sediments)
Natural wetlands (freshwater sediments)
Landfills
Tundra (freshwater sediments)
Anaerobic breakdown of organic matter.
Start with complex polymers
These are broken down into monomers by exo-enzymes
The exoenzymes are produced by fungi and bacteria
Monomers are fermented (fermentation because no oxygen and no other electron acceptors) into:
H2, CO2
Acetate
Propionate, butyrate, succinate, alcohols
The alcohols are broken down further by secondary fermentation. Which is syntropy. Producing H2, CO2 and Acetate
Acetogens turn the H2 and CO2, into acetate.
The last step is that methanogens convert everything into CH4 and CO2 by methanogenesis.
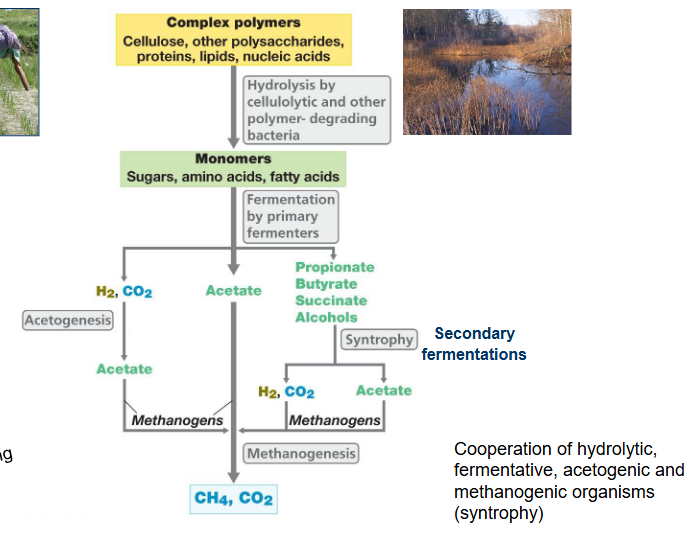
Syntrophy
Process in which at least two different microorganisms must work together to break down/use a compound: they cannot do it on their own.
For example one reaction has a positive delta G, and won’t happen by itself
Therefore it is combined with a reaction that has a negative delta G.
So that when they are combined the overall change in Gibbs energy is still negative.
Where can you find methanogens?
Termites
Ruminants
Rice fields
Puddles and marshes
Therefore these are large contributers to all the methane in the air.
What happens to methanogenesis in salt-water sediments/marine water?
Contains sulfate too that can accept electrons.
Therefore there is less methane produced because sulfate reduction is much more energy efficient.
There is a competition between methanogens and sulfate reducers.
The sulfite that is produced can also keep reacting with iron to make insoluble iron for example.
Methane hydrates
Methane ice
Methanotrophs
organisms which are able to obtain energy by oxidizing methane
Are dependent on methanogens
Require different environmental conditions compared to methanogens
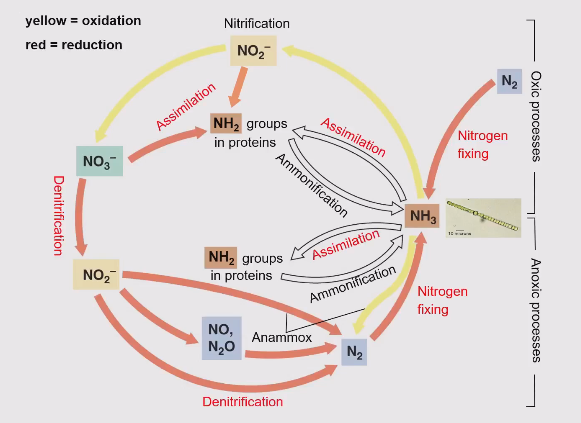
Ammonification
Organic N → NH4+
Nitrogen cycle
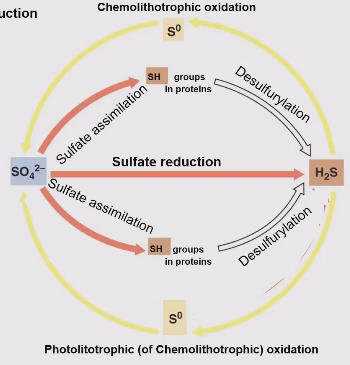
The sulfur cycle