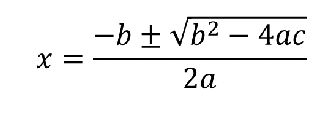CHEM 1112 Equilibrium
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Equilibrium
rate of the forward reaction is = to the rate of the reverse reaction
there is no net reaction in both directions
∆G = 0
no driving force for the forward or reverse process
the amounts of reactants and products are NOT usually equal to eachother at eqb’m
system NOT static at equilibrium
chemical eq’bm is a dynamic process
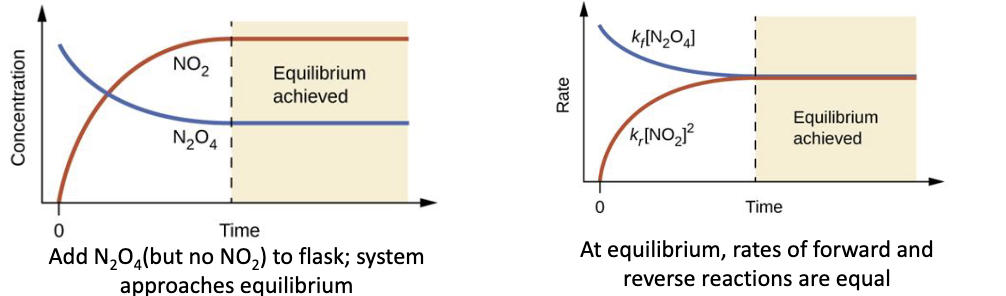
Dynamic Equilibrium (example)
N2O4 (g) → 2NO2 (g)
(colourless) (brown gas)
when N2O4 is placed in a closed container at 100*C, a reddish brown colour develops due to the formation of NO2
the forward reaction occurs
rate = kf[N2O4]
as NO2 builds up, it can also react to form N2O4
the reverse reaction occurs
rate= kr[NO2]²
at eqb’m, the amounts of reactant and products stop changing
reverse and forward reactions are equal
⇋ Eqb’m Arrows
the symbol ⇋ placed between reactants and products is used to designate reversible reactions
Reaction Quotient (Q)
allows us to mathematically express the amount of reactants and products present at any point in a reversable reaction
consider the general reaction: mA + nB ⇋ xC + yD
A, B, C, D are gases or aq sol’n
m, n, x, y are coefficients in balanced equations
Qc = [C]x[D]y /[A]m[B]n
WHY no liquids or solids
Qc and Keq are defined in terms of activity of a substance
concentration/standard concentration or pressure/standard pressure
the standard state for liquids/solids is the liquid/solid
the concentration of the liquid/solid does not change with the amount of a liquid/solid

Equilbrium Constant (Keq)
the value of Q when the reaction is at equilibrium
K calculated the same as Q…
consider the general reaction: mA + nB ⇋ xC + yD
A, B, C, D are gases or aq sol’n
m, n, x, y are coefficients in balanced equations
Qc = [C]x[D]y /[A]m[B]n
independent of the starting amounts of R and P
dependent on the temp. of the system
magnitude of K indicates the extent of a reaction
small k → mixture contains mostly R at eqb’m
large k → mixture contains mostly P at eqb’m
Q vs. K
K has the same form as Q, however, for K the concentrations MUST be at eqb’m, whereas for Q the concentrations can be those at any point in the reaction
when eqb’m is reached, Q = K
Equilibrium vs. Rxn Rate
the net rate of a reaction at equilibrium is 0
Adding k’s together
if a reaction can be expressed as the sum of two or more reactions, the eqb’m constants for the overall reaction is given by the product of the eqb’m constants of the individual reactions.
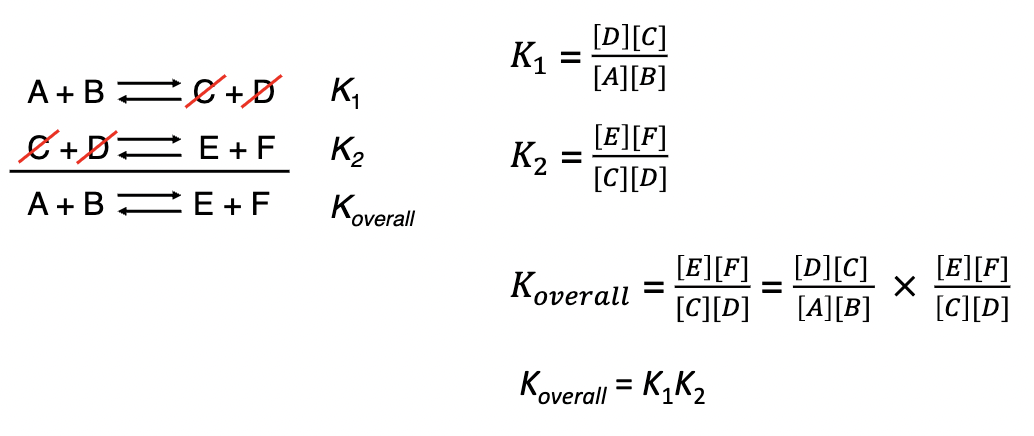
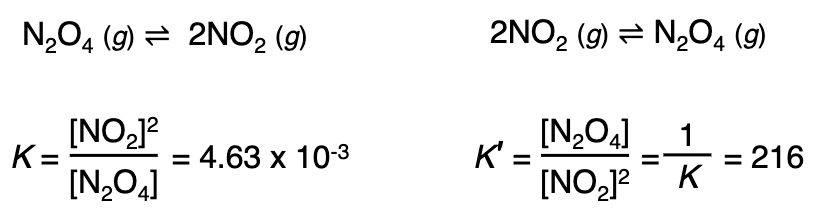
Reversing a k
when the equation for a reversible reaction is written in the opposite direction, the equilibrium constant becomes the reciprocal of the original eq’bm constant
Le Châtelier’s Principle
when a chemical system at eqb’m is disturbed, it returns to eqb’m by counteracting the disturbance
at eqb’m Q = K
the distance causes a change in Q
the reaction will shift to re-establish Q = K
Adding a R/P
if a chemical eqb’m is disturbed by adding a R or P, the system will proceed in the direction that consumes part of the added species
Removing a R/P
if a chemical eqb’m is disturbed by removing a R or P, the system will proceed in the direction that restores part of the removed species
Add/remove a pure liquid or solid
no effect on the system unless all of the liquid or solid is removed
because pure liquids and solids no not appear in the equilibrium expression
Changes in Temperature (le chat)
if you increase the temperature, equilibrium responds in fashion that consumes the added heat
the position of equilibrium will change
the value of k will change
recall that equilibrium constant will change if the temperature changes
if the forward reaction is EXOthermic, K decreases as T increases
if the forward reaction is ENDOthermic, K increases as T increases
Catalyst
a catalyst does not affect the equilibrium position
the position of equilibrium depends on ∆Grxn
∆G = RTlnK
a catalyst will increase the rate at which a reaction achieves equilibrium
k= Ae-Ea/RT
Effect of changes in volume of a gas
decreasing the volume of a gas increases the pressure, causing the reaction to shift to the right
fewer moles of gas, lower pressure
increasing the volume reduces the pressure, causing the reaction to shift to the left
more moles of gas, higher pressure
Relationship between Q and Keq
When we “disturb the equilibrium” by adding more reactant or product, Q does not = Keq
the system will react by trying to return to equilibrium, Q does not = Keq
the direction of the reaction will depend on if Q > Keq or Q < Keq
if Q < Keq the system shifts to the right in favour of the products
if Q = Keq the reaction is at eq’bm and there is no net change
if Q > Keq the system shifts to the left in favour of reactants
Gibbs Free Energy
∆G 0
helps us predict in which direction a reaction is spontaneous
if ∆G < 0, the reaction is spon in FORWARD direction
if ∆G = 0, the reaction is not spontaneous as system is at eq’bm
if ∆G > 0, the reaction is spon in the REVERSE direction
∆G and Q (or Keq)
∆Grxn = ∆Gorxn + RTlnQ
Q = reaction quotient
∆Gorxn = change in Gibbs Energy under standard conditions
∆Go = -RTlnKeq
∆Grxn = change in Gibbs Energy under actual conditions
if at eqb’m, ∆Grxn = 0 and Q= Keq
Calculating equilibirum constants
can measure the concentration or pressure of all reactants and products at eq’bm and insert into Kc expression
can measure the initial concentrations of reactants, and the equilibrium concentrations of ONLY one reactant
can deduce the other concentrations using ice tables
Quadratic equation