the energy change of reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what factor decides if a reaction is endothermic or exothermic?
Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break existing bonds and the energy released when the new bonds are formed
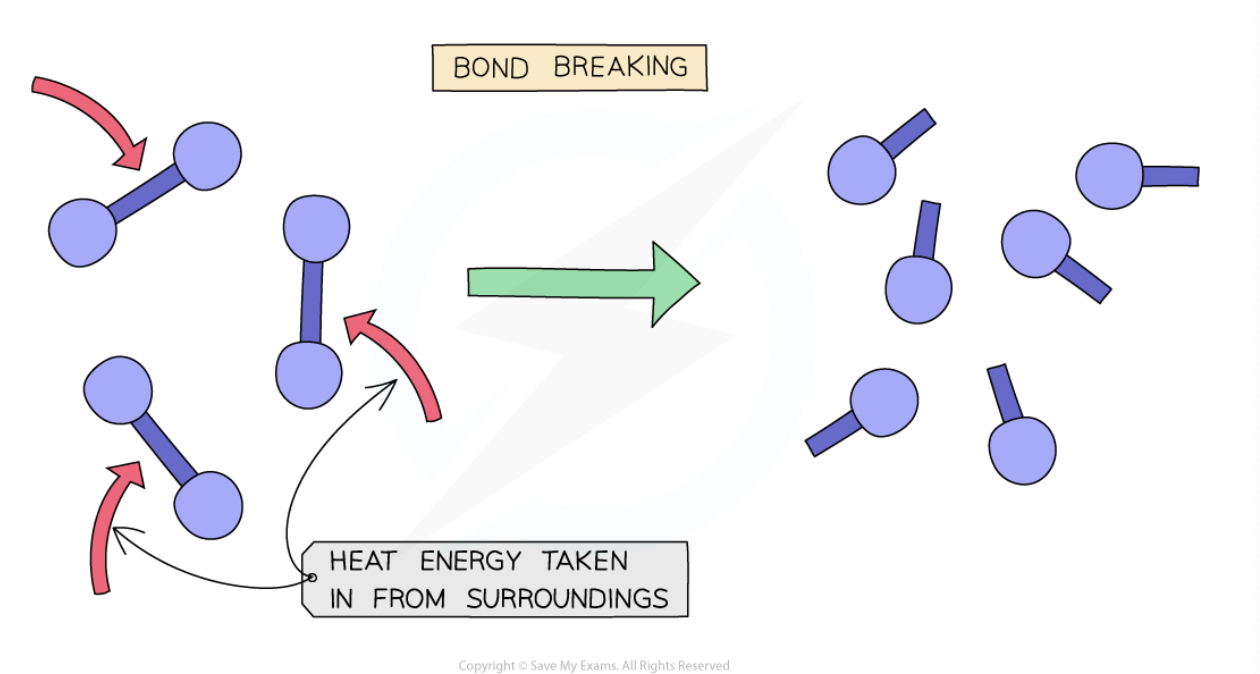
what process is bond breaking classified as? why?
Bond breaking is always an endothermic process as energy needs to be taken in from the surroundings to break the chemical bonds
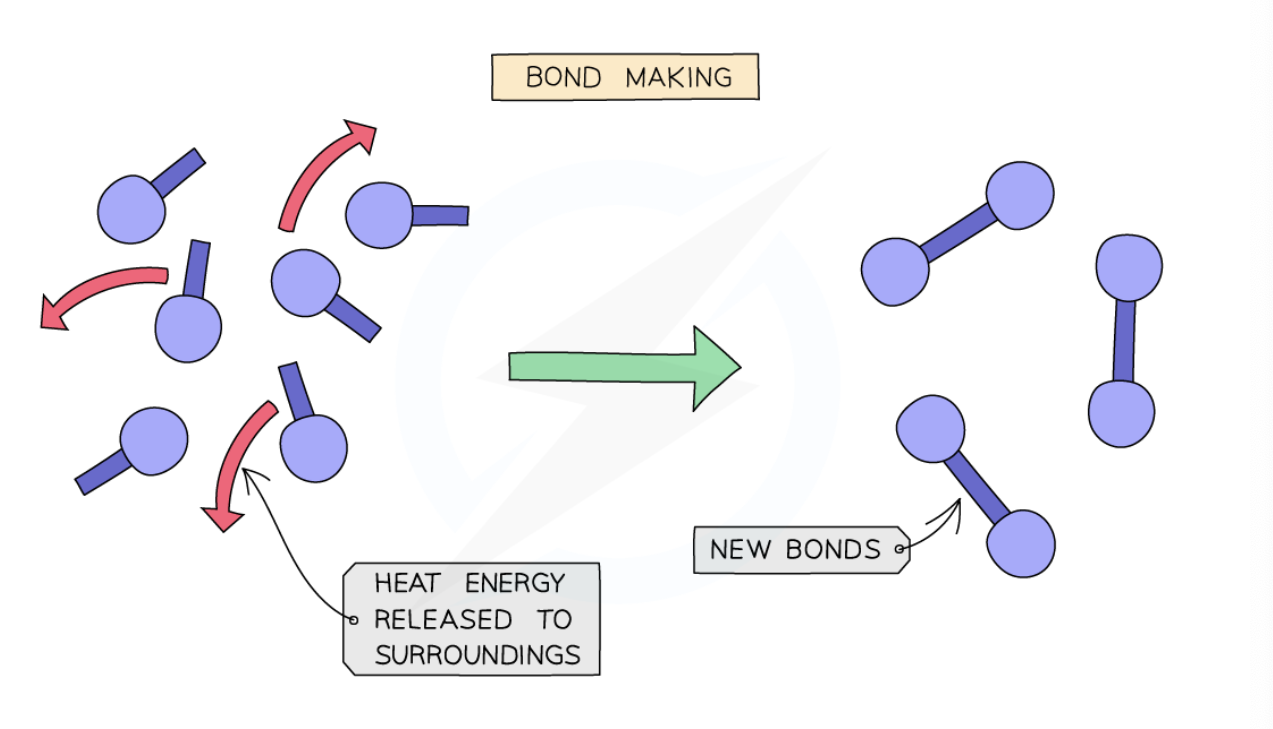
what process is bond making classified as? why?
Bond making is always an exothermic process as energy is transferred to the surroundings as the new bond is formed
what makes a reaction endothermic?
If more energy is absorbed than is released, this reaction is endothermic
what kind of change in energy do endothermic reactions have? why? (3)
More energy is required to break the bonds of reactant than is released from making the new bonds of the products
The change in energy is positive since the products have more energy than the reactants
Therefore an endothermic reaction has a positive change in energy
so when is an energy change positive?
energy change is positive if:
the products end up with more energy as they had to release less energy than what was needed to break the bonds of the reactants
more energy was absorbed than released
what is a requirement for bond breaking?
energy must be absorbed from the surroundings for bonds to be broken
when is a reaction exothermic?
If more energy is released than is absorbed, then the reaction is exothermic
why do exothermic reactions have a negative change in energy?
More energy is released when new bonds are formed than energy required to break the bonds in the reactants
The change in energy is negative since the products have less energy than the reactants
Therefore an exothermic reaction has a negative change in energy
what happens when new bonds are made when forming products?
Making new bonds gives off heat from the reaction to the surroundings
what does every chemical bond have?
Each chemical bond has a specific bond energy associated with it
what is bond energy?
the amount of energy required to break the bond or the amount of energy given out when the bond is formed
what can bond energy be used to calculate?
This energy can be used to calculate how much heat would be released or absorbed in a reaction
what do you need to know about reactants and products to calculate how much heat would be released/absorbed in a reaction?
know the bonds present in both the reactants and products
what can you calculate if you knowt he bond energies of all the species involved
total change in energy for a reaction
what are the three steps to calculating the total change in energy for a reaction
Add together all the bond energies for all the bonds in the reactants – this is the ‘energy in’
Add together the bond energies for all the bonds in the products – this is the ‘energy out’
Calculate the energy change using the equation:
Energy change = Energy taken in - Energy given out
what is the equation to calculate energy change?
Energy change = Energy taken in - Energy given out
what should you do before doing bond energy questions? (2)
write down a displayed formula equation for the reaction before identifying the type and number of bonds
take into account the balancing numbers when working out how many of each type of bond is being broken/formed