CHM2211- Ch. 19 IUPAC etc
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Carboxylic Acid (Substituent)
Carboxy
Carboxylic Acid (suffix)
-oic acid
Esters (Substituent)
alkoxycarbonyl
Ester (Suffix)
-oate
Acid Halides (Substituent)
halocarbonyl
Acid Halides (Suffix)
-oyl halide
Amides (Substituent)
carbamoyl
Amides (Suffix)
-amide
Nitriles (Substituent)
cyano
Nitriles (Suffix)
-nitrile
Aldehydes (Substituent)
oxo (formyl)
Aldehydes (Suffix)
-al
Ketones (Substituents)
oxo
Ketones (Suffix)
-one
Alcohols (Substituent)
hydroxy
Alcohols (Suffix)
-ol
Amines (Substituents)
amino
Amines (Suffix)
-amine
Ethers (Substituent)
alkoxy
Ethers (suffix)
-ethers
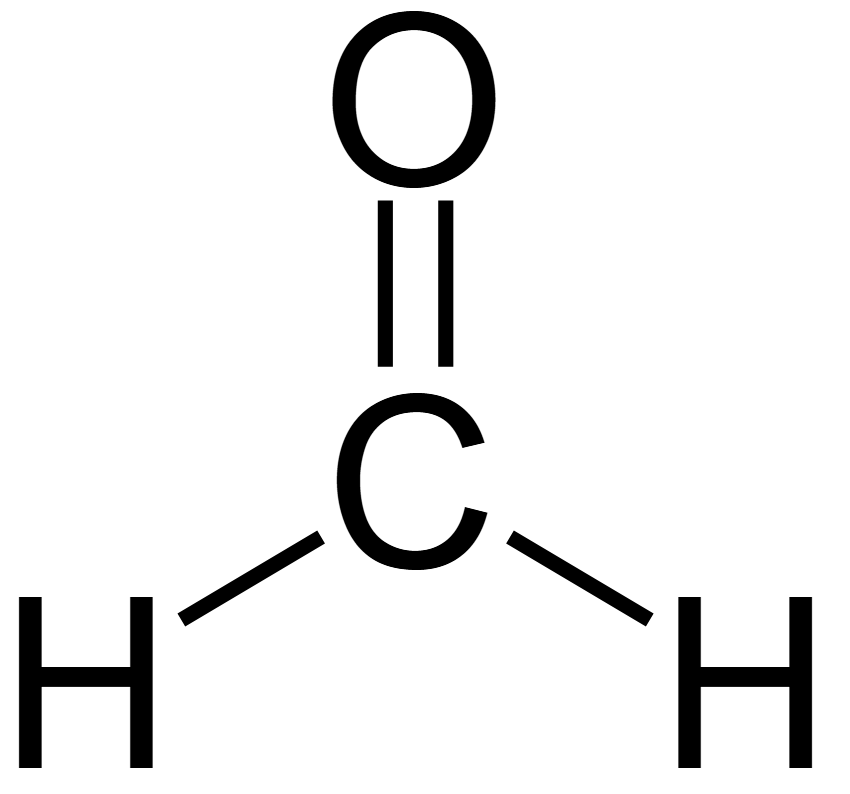
formaldehyde
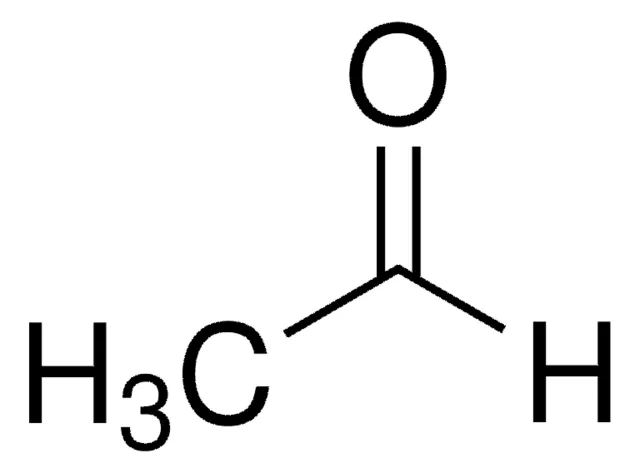
acetaldehyde
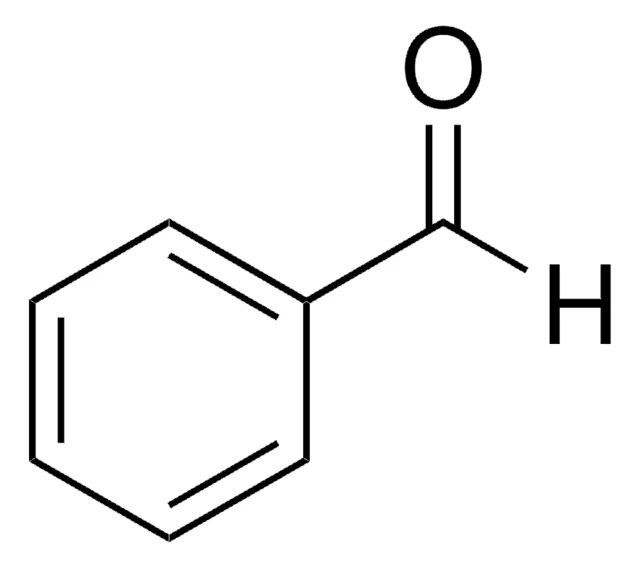
benzaldehyde
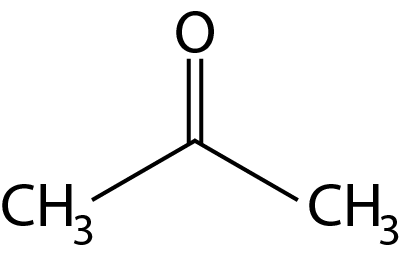
acetone
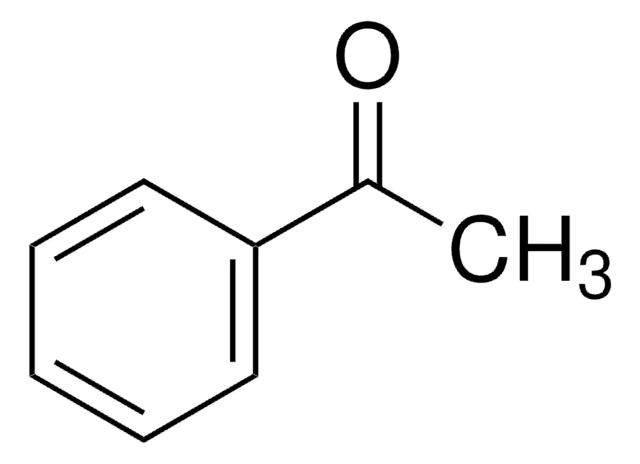
acetophenone
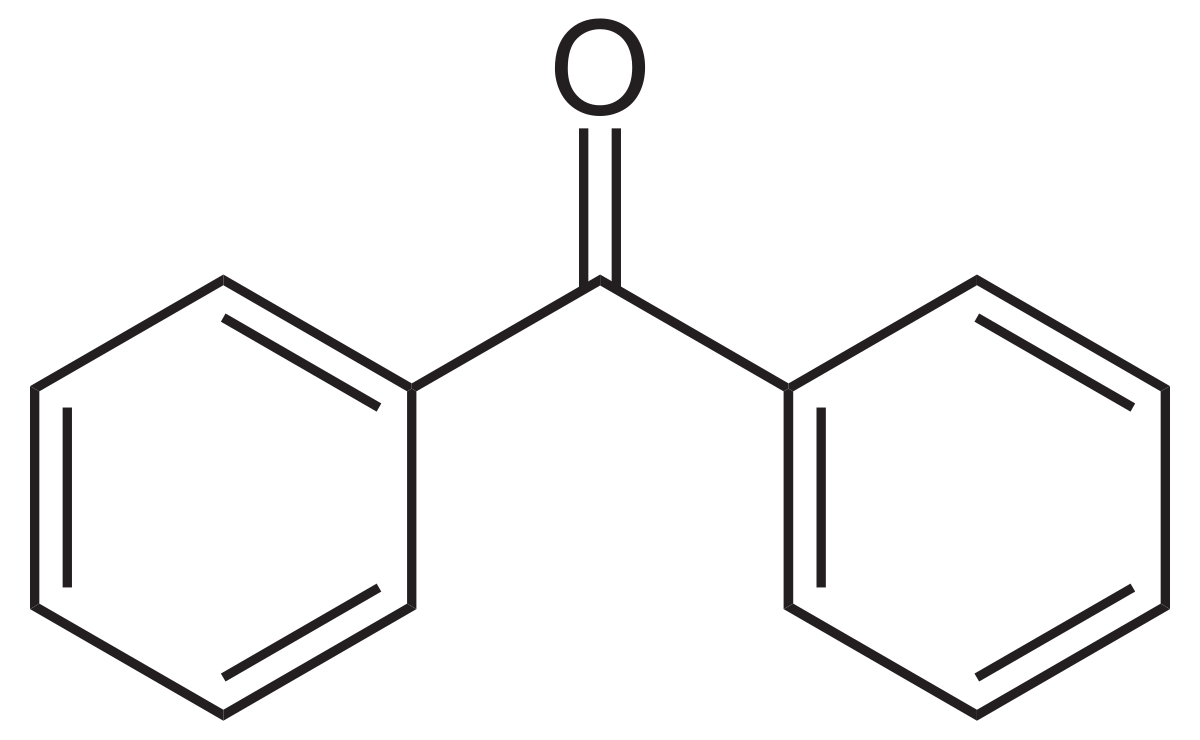
benzophenone
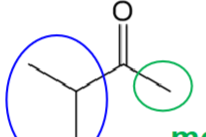
ketones can be named in ‘alkyl alkyl ketones’ order
isopropyl methyl ketone
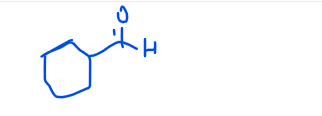
carbaldehyde
Why are aldehydes more reactive than ketones?
Steric hindrance and electronic effect. Carbonyl carbon in aldehyde has less EDG so the positive character is more enhanced, therefore a better electrophile for reactions.
Nucleophilic attack in basic condition
directly into a nucleophilic attack